Flow Rate Sensor inside Infusion Tube
Abstract
1. Introduction
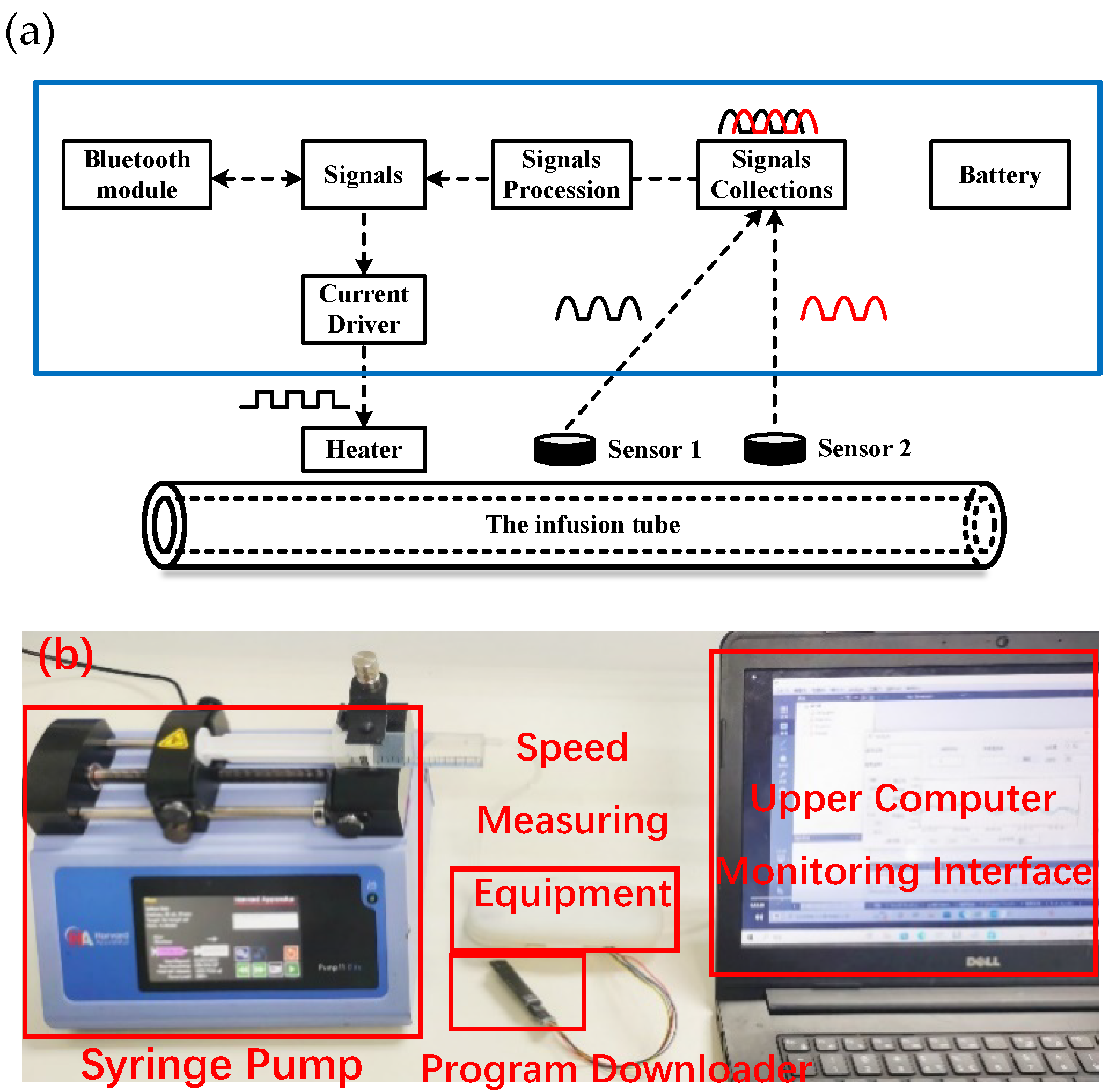
2. The Device Design of Flow Rate Sensor
2.1. The Experimental Scheme
2.2. The Flow Speed Calculation Modeling
2.2.1. Time-Domain Cross-Correlation Algorithms
2.2.2. Frequency-Domain Correlation Algorithms
2.2.3. The Relation of the Flow Speed and the Flow Rate
2.2.4. The Design of the Signal Process
3. The Performance of the Flow Rate Sensor Devices
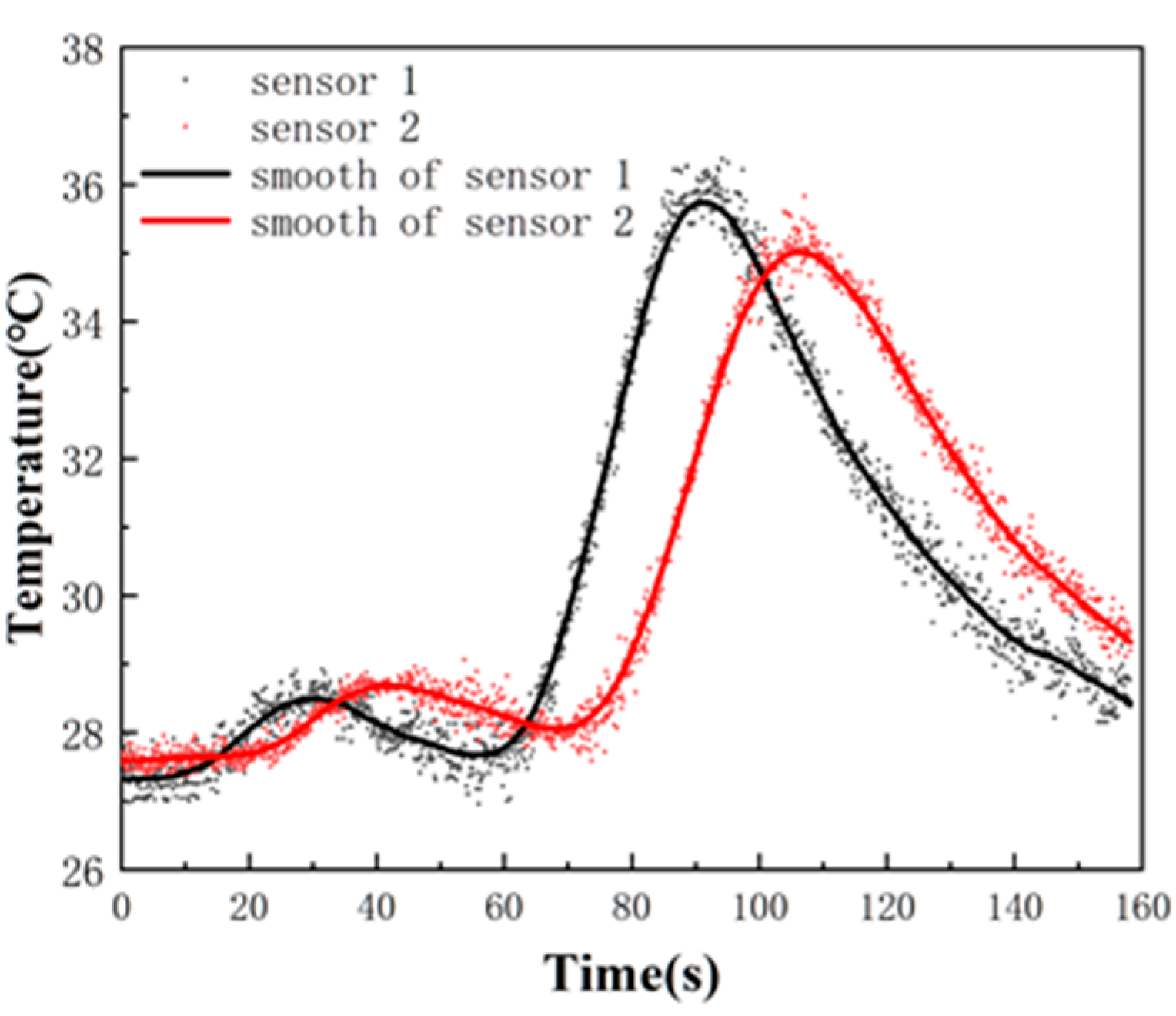
3.1. The Signals Process of the Flow Speed
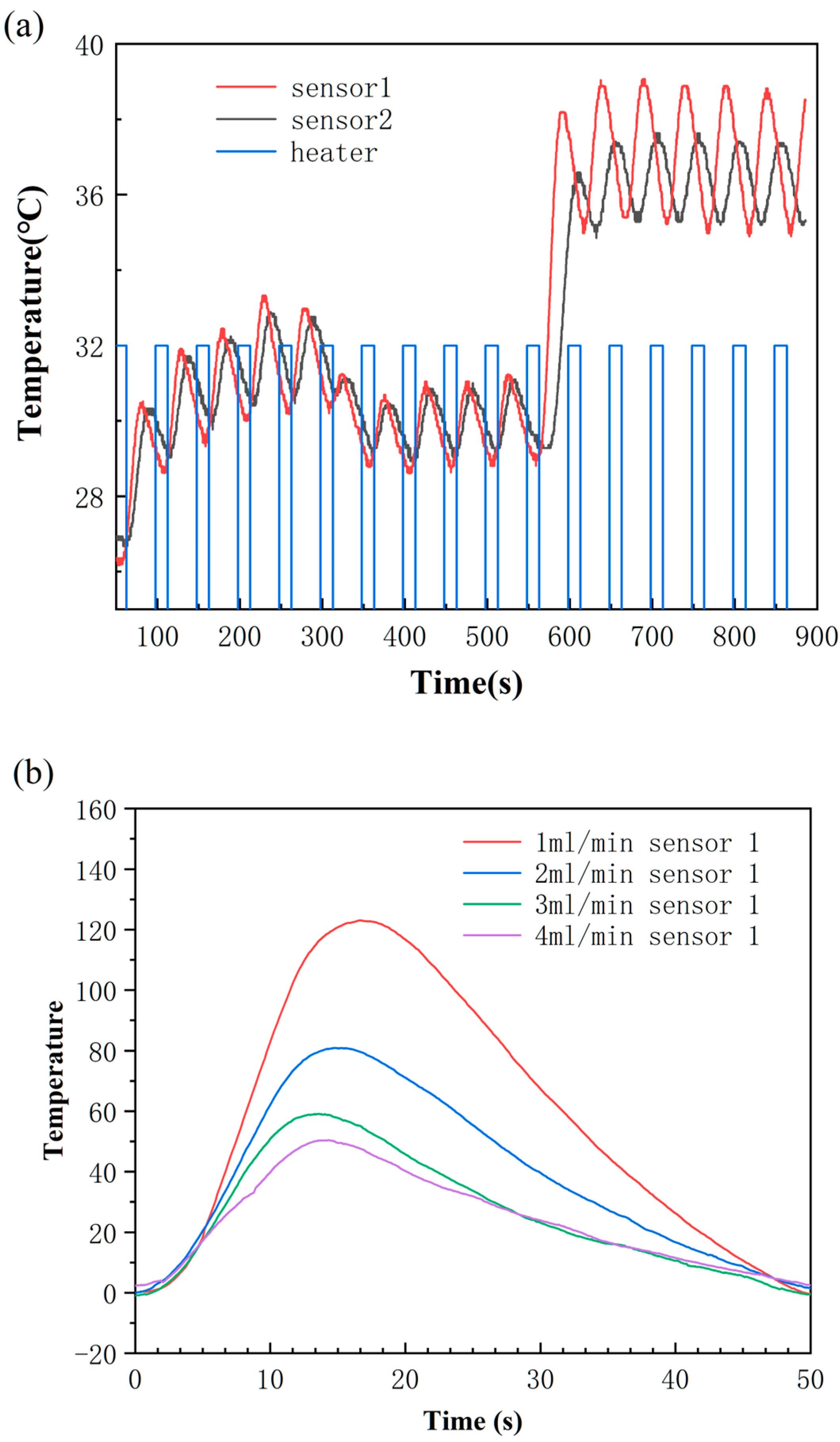
3.2. The Operation of This Flow Rate Sensor Device
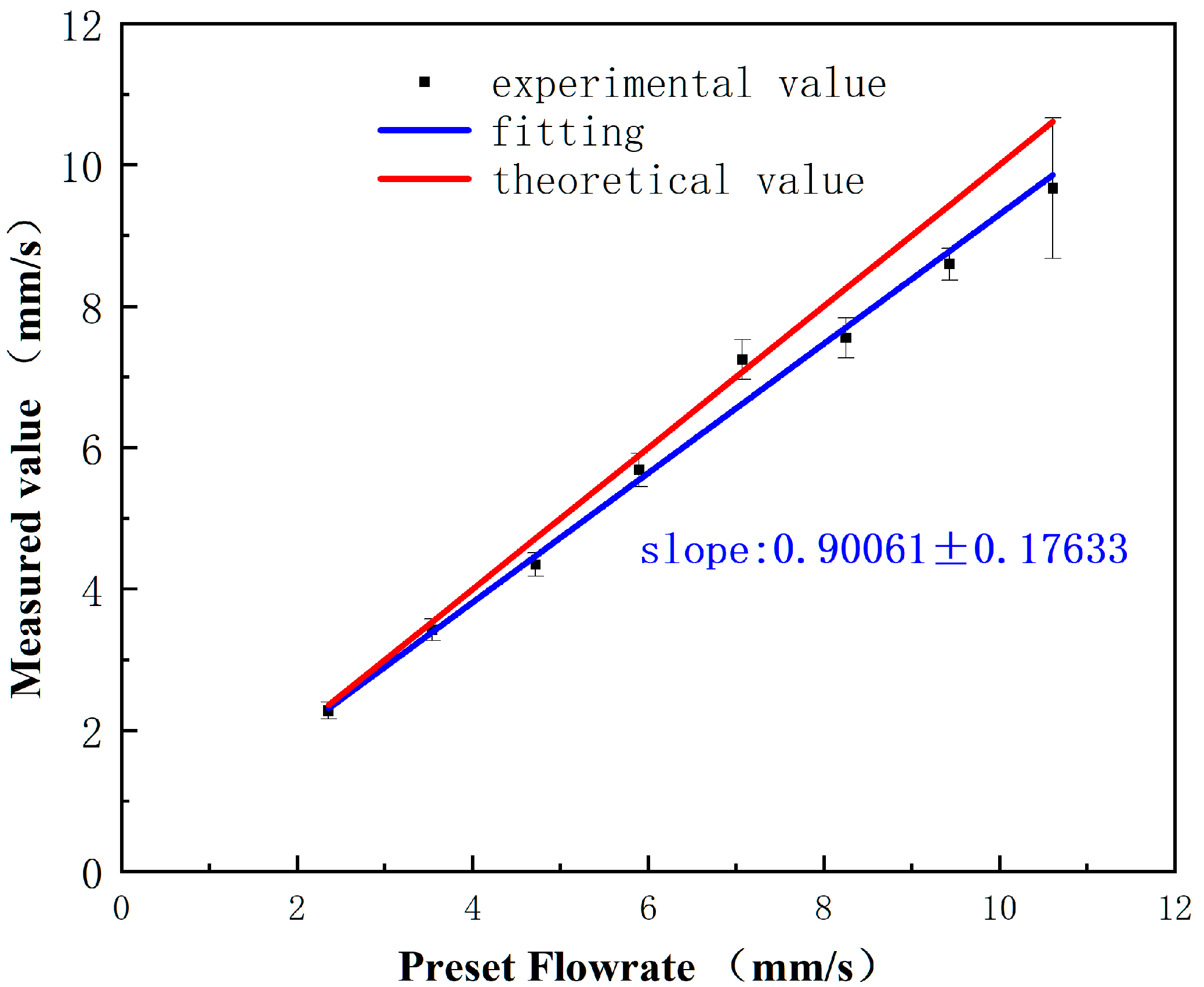
3.3. The Dynamic Range of This Flow Rate Sensor Device
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wakasa, Y.; Takada, K.; Yanagita, T. Reinforcing effect as a function of infusion speed in intravenous self-administration of nicotine in rhesus monkeys. Jpn. J. Psychopharmacol. 1995, 15, 53–59. [Google Scholar]
- Abreu, M.E.; Bigelow, G.E.; Fleisher, L.; Walsh, S.L. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology 2001, 154, 76–84. [Google Scholar] [CrossRef]
- Kendall, M.J.; Woods, K.L.; Wilkins, M.R.; Worthington, D.J. Responsiveness to beta-adrenergic receptor stimulation: The effects of age are cardioselective. Br. J. Clin. Pharmacol. 1982, 14, 821–826. [Google Scholar] [CrossRef]
- Kim, U.R.; Peterfreund, R.A.; Lovich, M.A. Drug Infusion Systems: Technologies, Performance, and Pitfalls. Anesth. Analg. 2017, 124, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Kohn, L.T.; Corrigan, J.M.; Donaldson, M.S. To Err Is Human: Building a Safer Health System; To Err is Human; Building a Safer Health System: Washington, DC, USA, 2000. [Google Scholar]
- Keohane, C.A.; Hayes, J.; Saniuk, C.; Rothschild, J.M.; Bates, D.W. Intravenous Medication Safety and Smart Infusion Systems. J. Infus. Nurs. 2005, 28, 321–328. [Google Scholar] [CrossRef]
- Zhang, Q.; Ruan, W.; Wang, H.; Zhou, Y.; Wang, Z.; Liu, L. A self-bended piezoresistive microcantilever flow sensor for low flow rate measurement. Sens. Actuators A Phys. 2010, 158, 273–279. [Google Scholar] [CrossRef]
- Nezhad, A.S.; Ghanbari, M.; Agudelo, C.G.; Packirisamy, M.; Bhat, R.B.; Geitmann, A. PDMS Microcantilever-Based Flow Sensor Integration for Lab-on-a-Chip. IEEE Sens. J. 2013, 13, 601–609. [Google Scholar] [CrossRef]
- Sadegh Cheri, M.; Latifi, H.; Sadeghi, J.; Salehi Moghaddam, M.; Shahraki, H.; Hajghassem, H. Real-time measurement of flow rate in microfluidic devices using a cantilever-based optofluidic sensor. Analyst 2014, 139, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Wenman, M.S. Method for Monitoring Infusion of Intravenous Fluid into a Patient. U.S. Patent No. 4,650,464, 17 March 1987. [Google Scholar]
- Chen, F.-G.; Wang, J.-Y.; Chen, S.; Tu, S.-C.; Chen, K.-Y. A Hang-and-Play Intravenous Infusion Monitoring System. In Proceedings of the 2015 3rd International Conference on Applied Computing and Information Technology/2nd International Conference on Computational Science and Intelligence, Okayama, Japan, 12–16 July 2015; pp. 278–281. [Google Scholar]
- Chiang, C.-T.; Huang, Y.-C. A Semicylindrical Capacitive Sensor With Interface Circuit Used for Flow Rate Measurement. IEEE Sens. J. 2006, 6, 1564–1570. [Google Scholar] [CrossRef]
- Cohen, L.; Rose, R.A. Capacitance-Type Fluid Level Sensor for i.v. and Catheter Bags. U.S. Patent No. 5,135,485, 4 August 1992. [Google Scholar]
- Wei, Q.; Lee, J.H.; Seong, K.W.; Kim, M.N.; Cho, J.H. The design of a wireless flexible capacitive sensor detection system to detect liquid level in plastic bag intravenous drip sets. Biomed. Eng. Lett. 2011, 1, 247–253. [Google Scholar] [CrossRef]
- Jianwen, C.; Han, Z. Design of intravenous infusion monitoring and alarm system based on wireless communication technology. In Proceedings of the IEEE International Conference Mechatronics & Automation, Beijing, China, 7–10 August 2011. [Google Scholar]
- Babu, C.G.; Kumar, J.R.D.; Balaji, V.R.; Priyadharsini, K.; Karthi, S.P. Performance Analysis of Smart Intravenous Infusion Systems using Machine Learning. In Proceedings of the 2021 Smart Technologies, Communication and Robotics (STCR), Sathyamangalam, India, 9–10 October 2021; pp. 1–7. [Google Scholar]
- Zhang, Y.; Zhang, S.; Ji, Y.; Wu, G. Intravenous infusion monitoring system based on WSN. In Proceedings of the International Conference on Wireless Sensor Network, Beijing, China, 15–17 November 2010. [Google Scholar]
- Deckert, C.; Wilson, L.L. Flow control system. U.S. Patent No. 4,681,563, 21 July 1987. [Google Scholar]
- Peng, D.; Cheng, Z.; Chen, H.; Li, Z.; Wei, Z. Development of infusion remote-control system based on wireless data-transfer and ultrasonic acquisition. Chin. Med. Equip. J. 2005, 8, 7–8. [Google Scholar]
- Mistry, K.K.; Mahapatra, A. Design and simulation of a thermo transfer type MEMS based micro flow sensor for arterial blood flow measurement. Microsyst. Technol. 2012, 18, 683–692. [Google Scholar] [CrossRef]
- Schnell, G. Measurement of flow in infusion systems. Med. Biol. Eng. Comput. 1997, 35, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, J.; Park, Y.; Lee, K.-H.; Kim, C.; Kwon, O.; Kim, S.; Lee, S.-R. Sensitive and reliable thermal micro-flow sensor for a drug infusion system. Sens. Actuators A Phys. 2020, 309, 112033. [Google Scholar] [CrossRef]
- Berthet, H.; Jundt, J.; Durivault, J.; Mercier, B.; Angelescu, D. Time-of-flight thermal flowrate sensor for lab-on-chip applications. Lab Chip 2011, 11, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Wang, Y.; Qian, W.; Wang, Y.; Qian, J. A self-powered spiral droplet triboelectric sensor for real-time monitoring of patient infusion in nursing wards. Nanotechnology 2024, 35, 155501. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, O.O.E.; Sunday, O.O.; Eyehorua, D.O. Development of automated intravenous blood infusion monitoring system using load cell sensor. J. Appl. Sci. Environ. Manag. 2018, 22, 1557–1561. [Google Scholar] [CrossRef]
- Tang, Z.; Tao, Z.; Cao, Y.; Zhang, Q.; Liu, W.; Cao, C. Capacitively coupled contactless conductivity detection-based sensor for liquid level measurement and application to clinical infusion monitoring. Sens. Actuators A Phys. 2024, 367, 115073. [Google Scholar] [CrossRef]
- Salmaz, U.; Ahsan, M.A.H.; Islam, T. High-Precision Capacitive Sensors for Intravenous Fluid Monitoring in Hospitals. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Lee, J.-K.; Yoon, K.-C.; Kim, K.G. Design of a Remote Monitoring System Based on Optical Sensors to Prevent Medical Accidents during Fluid Treatment. Appl. Sci. 2021, 11, 10124. [Google Scholar] [CrossRef]
- Venkatesh, K.; Alagundagi, S.S.; Garg, V.; Pasala, K.; Karia, D.; Arora, M. DripOMeter: An open-source opto-electronic system for intravenous (IV) infusion monitoring. HardwareX 2022, 12, e00345. [Google Scholar] [CrossRef] [PubMed]
- Alagundagi, S.S.; Pasala, K.; Arora, M. Opto-electronic system for intravenous infusion monitoring. In Proceedings of the 2018 10th International Conference on Communication Systems & Networks (COMSNETS), Bengaluru, India, 3–7 January 2018; pp. 688–692. [Google Scholar] [CrossRef]
- Wang, L. Design and study of a novel spatial pseudodepolarizer. Opt. Tech. 2014, 209–213. (In Chinese) [Google Scholar] [CrossRef]
- Cataldo, A.; Cannazza, G.; Giaquinto, N.; Trotta, A.; Andria, G. Development of a remote system for real-time control of intravenous drip infusions. In Proceedings of the 011 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 30–31 May 2011; pp. 234–237. [Google Scholar] [CrossRef]


| The Uncertainty Source | Uncertainty |
|---|---|
| Repeatability | 0.013 |
| Reproducibility | 0.007 |
| Stability | 0.003 |
| Instrumentation bias | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chui, H.-C.; Xu, Y.; Wang, Z.; Zhang, X.; Li, R.; Qin, K.-R. Flow Rate Sensor inside Infusion Tube. Inventions 2024, 9, 89. https://doi.org/10.3390/inventions9040089
Chui H-C, Xu Y, Wang Z, Zhang X, Li R, Qin K-R. Flow Rate Sensor inside Infusion Tube. Inventions. 2024; 9(4):89. https://doi.org/10.3390/inventions9040089
Chicago/Turabian StyleChui, Hsiang-Chen, Ying Xu, Zhiyuan Wang, Xianting Zhang, Rui Li, and Kai-Rong Qin. 2024. "Flow Rate Sensor inside Infusion Tube" Inventions 9, no. 4: 89. https://doi.org/10.3390/inventions9040089
APA StyleChui, H.-C., Xu, Y., Wang, Z., Zhang, X., Li, R., & Qin, K.-R. (2024). Flow Rate Sensor inside Infusion Tube. Inventions, 9(4), 89. https://doi.org/10.3390/inventions9040089







