Histological and Immunohistochemical Insights into Disc Perforation in the Temporomandibular Joint: A Case Report
Abstract
:1. Introduction
2. Materials and Methods
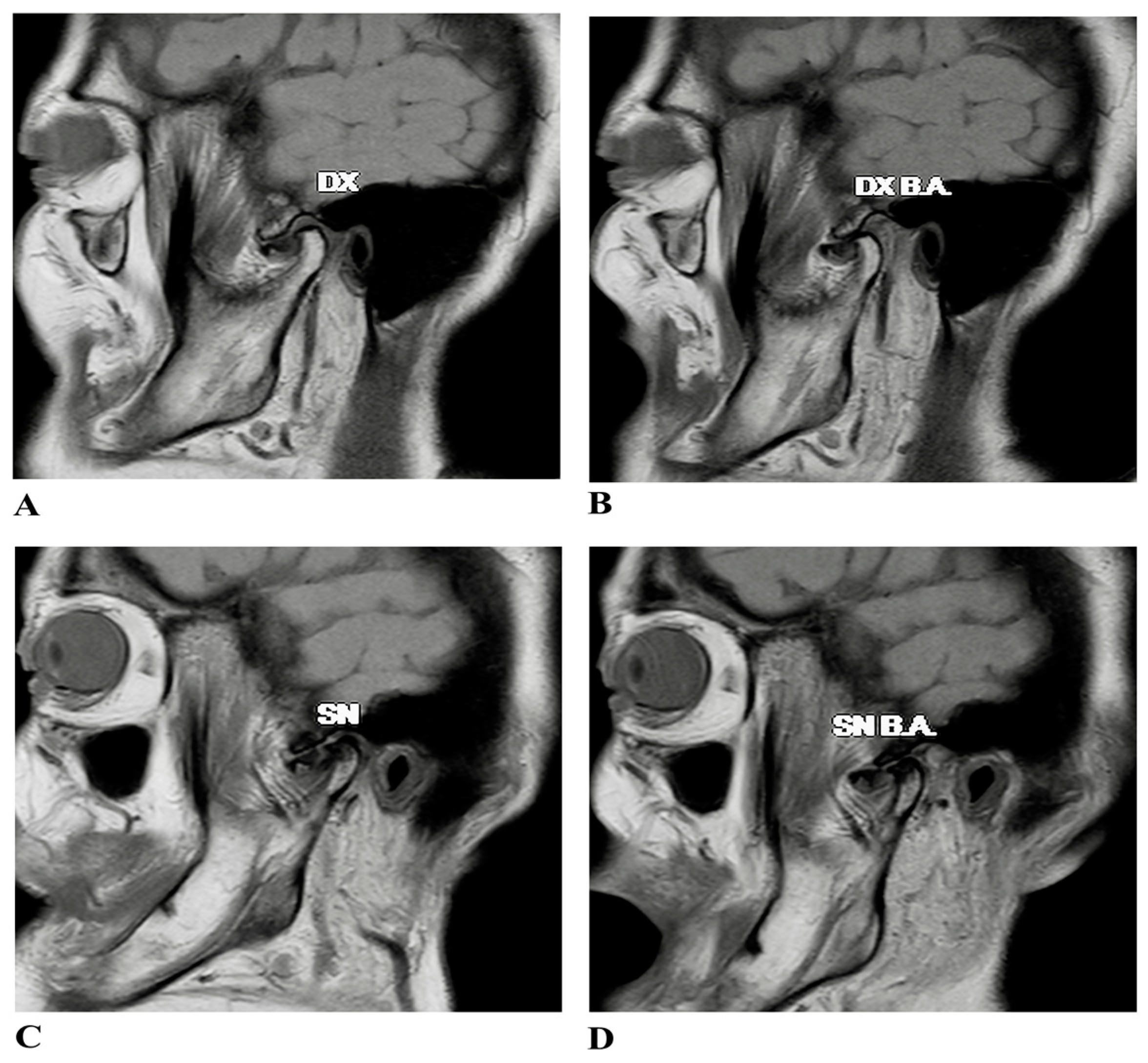
2.1. Case Presentation
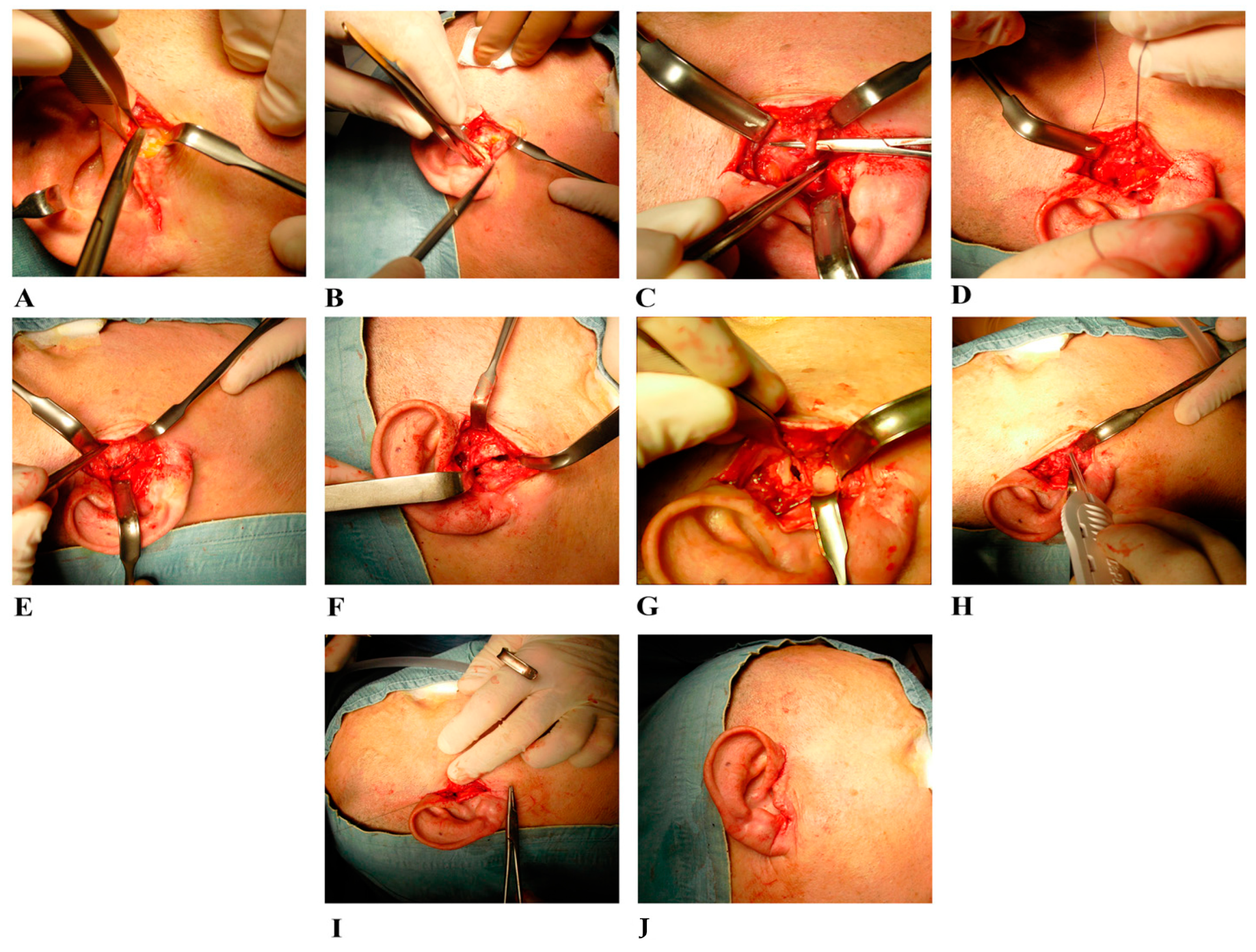
2.2. Surgical Treatment
2.3. Samples Collection
2.4. Light Microscopy
2.5. Immunofluorescence
3. Results
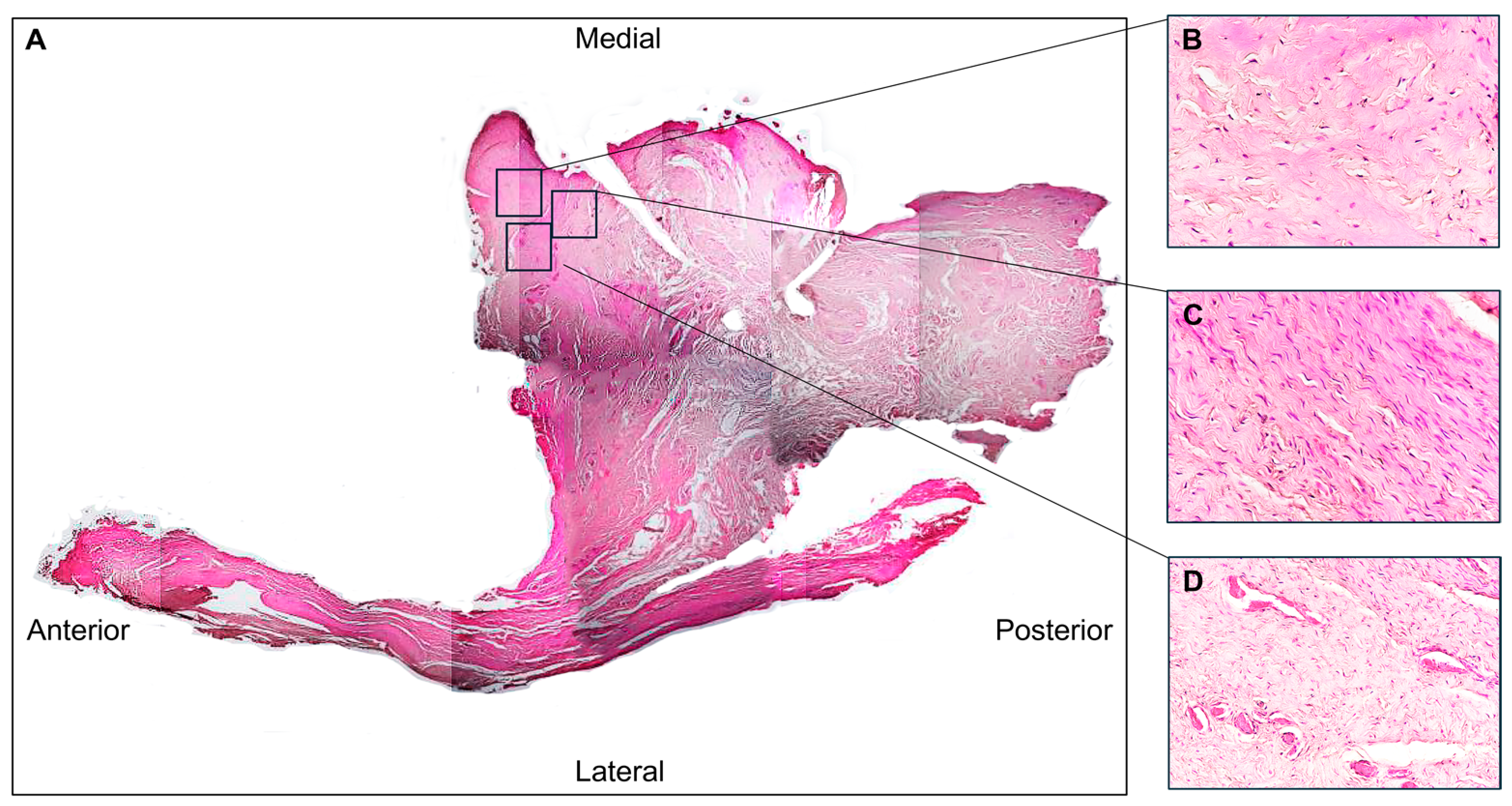
3.1. Histological Evaluation
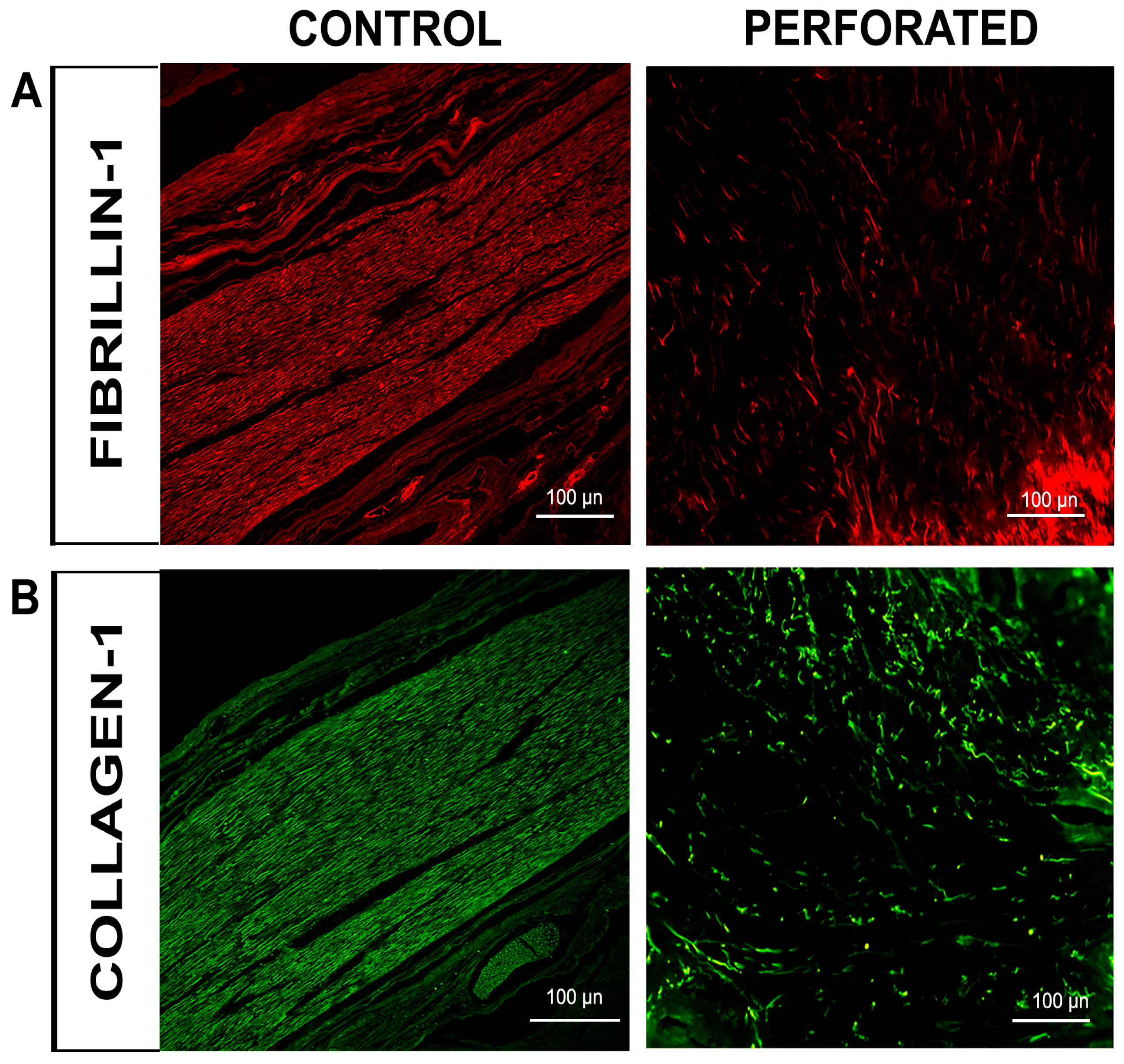
3.2. Immunofluorescence Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sindona, C.; Runci Anastasi, M.; Chiricosta, L.; Gugliandolo, A.; Silvestro, S.; Bramanti, P.; Cascone, P.; Mazzon, E. Temporomandibular Disorders Slow Down the Regeneration Process of Masticatory Muscles: Transcriptomic Analysis. Medicina 2021, 57, 354. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Dworkin, S.F. The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res. 2016, 95, 1093–1101. [Google Scholar] [CrossRef]
- Li, D.T.S.; Leung, Y.Y. Temporomandibular Disorders: Current Concepts and Controversies in Diagnosis and Management. Diagnostics 2021, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, D.F.; Faria-Teixeira, M.C.; Maffia, F.; Sanz, D.; Sarkis, M.; Marques, R.; Mota, B.; João, R.S.; Cardoso, H.J. Association of Malocclusion with Temporomandibular Disorders: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 4909. [Google Scholar] [CrossRef] [PubMed]
- Nastro, E.; Bonanno, L.; Catalfamo, L.; Runci, M.; Bramanti, A.; Anastasi, G.; Marino, S.; De Ponte, F.S. Diffusion Tensor Imaging Reveals Morphological Alterations of the Lateral Pterygoid Muscle in Patients with Mandibular Asymmetry. Dentomaxillofac. Radiol. 2018, 47, 20170129. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Miernik, M.; Więckiewicz, W. The Basic Conservative Treatment of Temporomandibular Joint Anterior Disc Displacement Without Reduction—Review. Adv. Clin. Exp. Med. 2015, 24, 731–735. [Google Scholar] [CrossRef]
- Stocum, D.L.; Roberts, W.E. Part I: Development and Physiology of the Temporomandibular Joint. Curr. Osteoporos. Rep. 2018, 16, 360–368. [Google Scholar] [CrossRef]
- Iturriaga, V.; Bornhardt, T.; Velasquez, N. Temporomandibular Joint: Review of Anatomy and Clinical Implications. Dent. Clin. N. Am. 2023, 67, 199–209. [Google Scholar] [CrossRef]
- Shah, M.A.; Deshmukh, K.S.; Dash, K.S.; Agrawal, S.; Mishra, A.; Kamath, V. Novel Techniques to Manage Temporomandibular Disorders—A Multidisciplinary Approach. J. Pharm. Bioallied Sci. 2024, 16, S3050–S3052. [Google Scholar] [CrossRef]
- Goodfred, J.; Simon, L.; Azam, A. Temporomandibular Junction Disorders. Prim. Care 2025, 52, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Runci Anastasi, M.; Macchi, V.; Vellone, V.; Nastro Siniscalchi, E.; Anastasi, G.; Morra, A.; Porzionato, A.; De Caro, R.; De Ponte, F.S.; Cascone, P. The Discomallear Ligament: Anatomical, Microscopical, and Radiologic Analysis. Surg. Radiol. Anat. 2020, 42, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Saczuk, K.; Kal, W.; Kaczała, A.; Wawrzeń, J.; Mielczarek, M.; Eyüboğlu, T.F.; Özcan, M.; Lukomska-Szymanska, M. The Coexistence of Tinnitus and Temporomandibular Disorder: A Narrative Review on the Importance of an Interdisciplinary Approach. J. Clin. Med. 2024, 13, 7346. [Google Scholar] [CrossRef]
- Mohlin, B.; Axelsson, S.; Paulin, G.; Pietilä, T.; Bondemark, L.; Brattström, V.; Hansen, K.; Holm, A.-K. TMD in Relation to Malocclusion and Orthodontic Treatment. Angle Orthod. 2007, 77, 542–548. [Google Scholar] [CrossRef]
- Chandwani, B.; Ceneviz, C.; Mehta, N.; Scrivani, S. Incidence of Bruxism in TMD Population. N. Y. State Dent. J. 2011, 77, 54–57. [Google Scholar]
- Sharma, S.; Gupta, D.S.; Pal, U.S.; Jurel, S.K. Etiological Factors of Temporomandibular Joint Disorders. Natl. J. Maxillofac. Surg. 2011, 2, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Karagah, T.; Aliabadi, E.; Hoseini, S.A. Does Gum Chewing Increase the Prevalence of Temporomandibular Disorders in Individuals with Gum Chewing Habits? J. Craniofac. Surg. 2014, 25, 1818–1821. [Google Scholar] [CrossRef]
- Incesu, L.; Taşkaya-Yılmaz, N.; Öğütcen-Toller, M.; Uzun, E. Relationship of Condylar Position to Disc Position and Morphology. Eur. J. Radiol. 2004, 51, 269–273. [Google Scholar] [CrossRef]
- Bae, W.C.; Biswas, R.; Statum, S.; Sah, R.L.; Chung, C.B. Sensitivity of Quantitative UTE MRI to the Biomechanical Property of the Temporomandibular Joint Disc. Skeletal Radiol. 2014, 43, 1217–1223. [Google Scholar] [CrossRef]
- Kang, H.; Bao, G.-J.; Qi, S.-N. Biomechanical Responses of Human Temporomandibular Joint Disc under Tension and Compression. Int. J. Oral Maxillofac. Surg. 2006, 35, 817–821. [Google Scholar] [CrossRef]
- Lai, L.; Huang, C.; Zhou, F.; Xia, F.; Xiong, G. Finite Elements Analysis of the Temporomandibular Joint Disc in Patients with Intra-Articular Disorders. BMC Oral Health 2020, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Zhang, S.Y.; Yang, C.; Chen, M.J.; YCai, X.; Haddad, M.S.; Yun, B.; Chen, Z.Z. Correlation between Disc Displacements and Locations of Disc Perforation in the Temporomandibular Joint. Dentomaxillofac. Radiol. 2010, 39, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Guerra, M.F.; Rodríguez-Campo, F.J.; Escorial Hernández, V.; Sánchez-Acedo, C.; Gil-Díez Usandizaga, J.L. Temporomandibular Joint Disc Perforation: Long-Term Results after Operative Arthroscopy. J. Oral Maxillofac. Surg. 2013, 71, 667–676. [Google Scholar] [CrossRef]
- Embree, M.C.; Iwaoka, G.M.; Kong, D.; Martin, B.N.; Patel, R.K.; Lee, A.H.; Nathan, J.M.; Eisig, S.B.; Safarov, A.; Koslovsky, D.A.; et al. Soft Tissue Ossification and Condylar Cartilage Degeneration Following TMJ Disc Perforation in a Rabbit Pilot Study. Osteoarthr. Cartil. 2015, 23, 629–639. [Google Scholar] [CrossRef]
- Roy, W.A. Temporomandibular Disorders: An Evidence-Based Approach to Diagnosis and Treatment. Phys. Ther. 2006, 86, 1451–1452. [Google Scholar] [CrossRef]
- Millon-Cruz, A.; Martín-Granizo, R.; Encinas, A.; Berguer, A. Relationship between Intra-Articular Adhesions and Disc Position in Temporomandibular Joints: Magnetic Resonance and Arthroscopic Findings and Clinical Results. J. Cranio-Maxillo-Facial Surg. 2015, 43, 497–502. [Google Scholar] [CrossRef]
- Machon, V.; Levorova, J.; Hirjak, D.; Drahos, M.; Foltan, R. Temporomandibular Joint Disc Perforation: A Retrospective Study. Int. J. Oral Maxillofac. Surg. 2017, 46, 1411–1416. [Google Scholar] [CrossRef]
- Shen, P.; Huo, L.; Zhang, S.Y.; Yang, C.; Cai, X.Y.; Liu, X.M. Magnetic Resonance Imaging Applied to the Diagnosis of Perforation of the Temporomandibular Joint. J. Cranio-Maxillo-Facial Surg. 2014, 42, 874–878. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jeon, K.-J.; Kim, M.-G.; Park, K.-H.; Huh, J.-K. A Nomogram for Classification of Temporomandibular Joint Disk Perforation Based on Magnetic Resonance Imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 682–692. [Google Scholar] [CrossRef]
- Gil, C.; Santos, K.C.P.; Dutra, M.E.P.; Kodaira, S.K.; Oliveira, J.X. MRI Analysis of the Relationship between Bone Changes in the Temporomandibular Joint and Articular Disc Position in Symptomatic Patients. Dentomaxillofac. Radiol. 2012, 41, 367–372. [Google Scholar] [CrossRef]
- Arayasantiparb, R.; Tsuchimochi, M. Quantification of Disc Displacement in Internal Derangement of the Temporomandibular Joint Using Magnetic Resonance Imaging. Odontology 2010, 98, 73–81. [Google Scholar] [CrossRef]
- Orhan, K.; Seki, U.; Rozylo-Kalinowska, I. Diagnostic Accuracy of Magnetic Resonance Imaging and Clinical Signs of Temporomandibular Joint Disorders: A 10-Year Research Update Review. Oral Radiol. 2017, 33, 81–91. [Google Scholar] [CrossRef]
- Kuribayashi, A.; Okochi, K.; Kobayashi, K.; Kurabayashi, T. MRI Findings of Temporomandibular Joints with Disk Perforation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 419–425. [Google Scholar] [CrossRef]
- Yura, S.; Nobata, K.; Shima, T. Diagnostic Accuracy of Fat-Saturated T2-Weighted Magnetic Resonance Imaging in the Diagnosis of Perforation of the Articular Disc of the Temporomandibular Joint. Br. J. Oral Maxillofac. Surg. 2012, 50, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Venetis, G.; Pilavaki, M.; Triantafyllidou, K.; Papachristodoulou, A.; Lazaridis, N.; Palladas, P. The Value of Magnetic Resonance Arthrography of the Temporomandibular Joint in Imaging Disc Adhesions and Perforations. Dentomaxillofac. Radiol. 2011, 40, 84–90. [Google Scholar] [CrossRef]
- Gao, W.; Lu, J.; Gao, X.; Zhou, J.; Dai, H.; Sun, M.; Xu, J. Biomechanical Effects of Joint Disc Perforation on the Temporomandibular Joint: A 3D Finite Element Study. BMC Oral Health 2023, 23, 943. [Google Scholar] [CrossRef]
- De Rossi, S.S.; Greenberg, M.S.; Liu, F.; Steinkeler, A. Temporomandibular Disorders: Evaluation and Management. In Medical Clinics of North America; W.B. Saunders: Philadelphia, PA, USA, 2014; Volume 98, pp. 1353–1384. [Google Scholar]
- Israel, H.A. Intra-Articular Operative Temporomandibular Joint Arthroscopy. Front. Oral Maxillofac. Med. 2020, 3. [Google Scholar] [CrossRef]
- Yang, R.; Lee, L.M.; Zhu, Y.; Jia, W.Y.; Yao, W.; Yu, Y.; Wu, S.J. Correlation Between Temporomandibular Joint Disc Perforation and Degenerative Joint Changes: A CBCT and Clinical Analysis. J. Oral Rehabil. 2024, 51, 2675–2682. [Google Scholar] [CrossRef]
- Espinoza, S.; Varela, D.; Richter, C.; Sepúlveda, G.; Marfull, N. Reproducibility of the Rocabado Pain Map. Cranio 2023, 41, 112–118. [Google Scholar] [CrossRef]
- Cascone, P.; Spallaccia, F.; Vellone, V. Temporomandibular Joint Surgery: Open Discopexy and “Functional Arthroplasty”. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2022, 30, 193–198. [Google Scholar] [CrossRef]
- Antonuccio, P.; Pallio, G.; Marini, H.R.; Irrera, N.; Romeo, C.; Puzzolo, D.; Freni, J.; Santoro, G.; Pirrotta, I.; Squadrito, F.; et al. Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium. Int. J. Mol. Sci. 2022, 23, 13144. [Google Scholar] [CrossRef] [PubMed]
- Freni, J.; Pallio, G.; Marini, H.R.; Micali, A.; Irrera, N.; Romeo, C.; Puzzolo, D.; Mannino, F.; Minutoli, L.; Pirrotta, I.; et al. Positive Effects of the Nutraceutical Association of Lycopene and Selenium in Experimental Varicocele. Int. J. Mol. Sci. 2023, 24, 13526. [Google Scholar] [CrossRef] [PubMed]
- Urzì Brancati, V.; Aliquò, F.; Freni, J.; Pantano, A.; Galipò, E.; Puzzolo, D.; Minutoli, L.; Marini, H.R.; Campo, G.M.; D’Ascola, A. The Effects of Seleno-Methionine in Cadmium-Challenged Human Primary Chondrocytes. Pharmaceuticals 2024, 17, 936. [Google Scholar] [CrossRef]
- Vermiglio, G.; Centofanti, A.; Matarese, G.; Militi, A.; Matarese, M.; Arco, A.; Nicita, F.; Cutroneo, G. Human Dental Pulp Tissue during Orthodontic Tooth Movement: An Immunofluorescence Study. J. Funct. Morphol. Kinesiol. 2020, 5, 65. [Google Scholar] [CrossRef]
- Runci Anastasi, M.; Centofanti, A.; Arco, A.; Vermiglio, G.; Nicita, F.; Santoro, G.; Cascone, P.; Anastasi, G.P.; Rizzo, G.; Cutroneo, G. Histological and Immunofluorescence Study of Discal Ligaments in Human Temporomandibular Joint. J. Funct. Morphol. Kinesiol. 2020, 5, 90. [Google Scholar] [CrossRef]
- Anastasi, M.R.; Centofanti, A.; Favaloro, A.; Freni, J.; Nicita, F.; Vermiglio, G.; Anastasi, G.P.; Cascone, P. Unilateral “Inactive” Condylar Hyperplasia: New Histological Data. J. Funct. Morphol. Kinesiol. 2024, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Vermiglio, G.; Centofanti, A.; Ramieri, G.; Tepedino, M.; Runci Anastasi, M.; Micali, A.G.; Arco, A.; Piancino, M.G. Immunofluorescence Evaluation of Myf5 and MyoD in Masseter Muscle of Unilateral Posterior Crossbite Patients. J. Funct. Morphol. Kinesiol. 2020, 5, 80. [Google Scholar] [CrossRef]
- Tanaka, E.; Koolstra, J.H. Biomechanics of the Temporomandibular Joint. J. Dent. Res. 2008, 87, 989–991. [Google Scholar] [CrossRef]
- Fazaeli, S.; Ghazanfari, S.; Everts, V.; Smit, T.H.; Koolstra, J.H. The Contribution of Collagen Fibers to the Mechanical Compressive Properties of the Temporomandibular Joint Disc. Osteoarthr. Cartil. 2016, 24, 1292–1301. [Google Scholar] [CrossRef]
- Fazaeli, S.; Ghazanfari, S.; Mirahmadi, F.; Everts, V.; Smit, T.H.; Koolstra, J.H. The Dynamic Mechanical Viscoelastic Properties of the Temporomandibular Joint Disc: The Role of Collagen and Elastin Fibers from a Perspective of Polymer Dynamics. J. Mech. Behav. Biomed. Mater. 2019, 100, 103406. [Google Scholar] [CrossRef]
- Kuo, J.; Zhang, L.; Bacro, T.; Yao, H. The Region-Dependent Biphasic Viscoelastic Properties of Human Temporomandibular Joint Discs under Confined Compression. J. Biomech. 2010, 43, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Barlattani, A.J.; Martelli, M.; Gargari, M.; Ottria, L. Articular Disc of Temporomandibular Joint: An Anatomical and Histological Study. Functional Considerations. J. Biol. Regul. Homeost. Agents 2019, 33, 199–208. [Google Scholar]
- Sbardella, D.; Fasciglione, G.F.; Gioia, M.; Ciaccio, C.; Tundo, G.R.; Marini, S.; Coletta, M. Human Matrix Metalloproteinases: An Ubiquitarian Class of Enzymes Involved in Several Pathological Processes. Mol. Aspects Med. 2012, 33, 119–208. [Google Scholar] [CrossRef]
- Fujita, H.; Morisugi, T.; Tanaka, Y.; Kawakami, T.; Kirita, T.; Yoshimura, Y. MMP-3 Activation Is a Hallmark Indicating an Early Change in TMJ Disorders, and Is Related to Nitration. Int. J. Oral Maxillofac. Surg. 2009, 38, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.E.; Caporal, K.; Ambros, V.; Azevedo, M.; Noronha, L.; Leonardi, R.; Trevilatto, P.C. Immunohistochemical Expression of Matrix Metalloprotease-2 and Matrix Metalloprotease-9 in the Disks of Patients with Temporomandibular Joint Dysfunction. J. Oral Pathol. Med. 2015, 44, 75–79. [Google Scholar] [CrossRef]
- Loreto, C.; Leonardi, R.; Musumeci, G.; Pannone, G.; Castorina, S. An Ex Vivo Study on Immunohistochemical Localization of MMP-7 and MMP-9 in Temporomandibular Joint Discs with Internal Derangement. Eur. J. Histochem. 2013, 57, e12. [Google Scholar] [CrossRef]
- Feng, Y.; Ke, J.; Cao, P.; Deng, M.; Li, J.; Cai, H.; Meng, Q.; Li, Y.; Long, X. HMGB1-Induced Angiogenesis in Perforated Disc Cells of Human Temporomandibular Joint. J. Cell. Mol. Med. 2018, 22, 1283–1291. [Google Scholar] [CrossRef]
- Loreto, C.; Almeida, L.E.; Trevilatto, P.; Leonardi, R. Apoptosis in Displaced Temporomandibular Joint Disc with and without Reduction: An Immunohistochemical Study. J. Oral Pathol. Med. 2011, 40, 103–110. [Google Scholar] [CrossRef]
- Luo, P.; Feng, C.; Jiang, C.; Ren, X.; Gou, L.; Ji, P.; Xu, J. IL-37b Alleviates Inflammation in the Temporomandibular Joint Cartilage via IL-1R8 Pathway. Cell Prolif. 2019, 52, e12692. [Google Scholar] [CrossRef] [PubMed]
- Runci Anastasi, M.; Cascone, P.; Anastasi, G.P.; Santoro, G.; Nicita, F.; Picciolo, G.; Favaloro, A.; Rizzo, G.; Cutroneo, G. Articular Disc of a Human Temporomandibular Joint: Evaluation through Light Microscopy, Immunofluorescence and Scanning Electron Microscopy. J. Funct. Morphol. Kinesiol. 2021, 6, 22. [Google Scholar] [CrossRef]
- Jiang, N.; Tan, P.; Sun, Y.; Zhou, J.; Ren, R.; Li, Z.; Zhu, S. Microstructural, Micromechanical Atlas of the Temporomandibular Joint Disc. J. Dent. Res. 2024, 103, 555–564. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Sun, Y.; Jiang, N. The Mechanics of Tissue-Engineered Temporomandibular Joint Discs: Current Status and Prospects for Enhancement. J. Biomater. Appl. 2024, 39, 269–287. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freni, J.; Centofanti, A.; Nicita, F.; Labellarte, D.; Vermiglio, G.; Anastasi, M.R. Histological and Immunohistochemical Insights into Disc Perforation in the Temporomandibular Joint: A Case Report. J. Funct. Morphol. Kinesiol. 2025, 10, 107. https://doi.org/10.3390/jfmk10020107
Freni J, Centofanti A, Nicita F, Labellarte D, Vermiglio G, Anastasi MR. Histological and Immunohistochemical Insights into Disc Perforation in the Temporomandibular Joint: A Case Report. Journal of Functional Morphology and Kinesiology. 2025; 10(2):107. https://doi.org/10.3390/jfmk10020107
Chicago/Turabian StyleFreni, Josè, Antonio Centofanti, Fabiana Nicita, Davide Labellarte, Giovanna Vermiglio, and Michele Runci Anastasi. 2025. "Histological and Immunohistochemical Insights into Disc Perforation in the Temporomandibular Joint: A Case Report" Journal of Functional Morphology and Kinesiology 10, no. 2: 107. https://doi.org/10.3390/jfmk10020107
APA StyleFreni, J., Centofanti, A., Nicita, F., Labellarte, D., Vermiglio, G., & Anastasi, M. R. (2025). Histological and Immunohistochemical Insights into Disc Perforation in the Temporomandibular Joint: A Case Report. Journal of Functional Morphology and Kinesiology, 10(2), 107. https://doi.org/10.3390/jfmk10020107







