Relationship Between Systemic Inflammation and Glycemic Control in Firefighters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Protocol
2.3.1. Body Composition and Cardiovascular Health Assessment
2.3.2. Venous Blood Measure
HbA1c Analysis
Glucose Analysis
Insulin Analysis
CRP Analysis
Homocysteine Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Gupta, M.K.; Gouda, G.; Vadde, R. Relation Between Obesity and Type 2 Diabetes: Evolutionary Insights, Perspectives and Controversies. Curr. Obes. Rep. 2024, 13, 475–495. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Beckett, A.; Scott, J.R.; Chater, A.M.; Ferrandino, L.; Aldous, J.W.F. The Prevalence of Metabolic Syndrome and Its Components in Firefighters: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6814. [Google Scholar] [CrossRef]
- Barros, B.; Oliveira, M.; Morais, S. Firefighters’ occupational exposure: Contribution from biomarkers of effect to assess health risks. Environ. Int. 2021, 156, 106704. [Google Scholar] [CrossRef] [PubMed]
- Superko, H.R.; Momary, K.M.; Pendyala, L.K.; Williams, P.T.; Frohwein, S.; Garrett, B.C.; Skrifvars, C.; Gadesam, R.; King, S.B., 3rd; Rolader, S.; et al. Firefighters, heart disease, and aspects of insulin resistance: The FEMA Firefighter Heart Disease Prevention study. J. Occup. Environ. Med. 2011, 53, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Cui, C.L.; Wu, P.; Xie, N.Z. Relationship of serum homocysteine level with nutritional status and HbA1c level in elderly inpatients. Int. J. Clin. Exp. Med. 2013, 6, 779–784. [Google Scholar]
- Chen, J.; Muntner, P.; Hamm, L.L.; Fonseca, V.; Batuman, V.; Whelton, P.K.; He, J. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J. Am. Soc. Nephrol. 2003, 14, 469–477. [Google Scholar] [CrossRef]
- Mossmann, M.; Wainstein, M.V.; Gonçalves, S.C.; Wainstein, R.V.; Gravina, G.L.; Sangalli, M.; Veadrigo, F.; Matte, R.; Reich, R.; Costa, F.G.; et al. HOMA-IR is associated with significant angiographic coronary artery disease in non-diabetic, non-obese individuals: A cross-sectional study. Diabetol. Metab. Syndr. 2015, 7, 100. [Google Scholar] [CrossRef]
- Richardson, A.; Terrazzini, N.; Gage, C.; Lee, B.J.; Bradley, R.; Watt, P.; Watkins, E.R. Inflammatory and psychological consequences of chronic high exposure firefighting. J. Therm. Biol. 2023, 111, 103399. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Guardado Mendoza, R.; Winnier, D.; Fiorentino, T.V.; Pengou, Z.; Cornell, J.; Andreozzi, F.; Jenkinson, C.; Cersosimo, E.; Federici, M.; et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol. 2014, 51, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Saito, I.; Folsom, A.R.; Brancati, F.L.; Duncan, B.B.; Chambless, L.E.; McGovern, P.G. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Ann. Intern. Med. 2000, 133, 81–91. [Google Scholar] [CrossRef]
- Best, L.G.; Zhang, Y.; Lee, E.T.; Yeh, J.L.; Cowan, L.; Palmieri, V.; Roman, M.; Devereux, R.B.; Fabsitz, R.R.; Tracy, R.P.; et al. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: The Strong Heart Study. Circulation 2005, 112, 1289–1295. [Google Scholar] [CrossRef]
- Soinio, M.; Marniemi, J.; Laakso, M.; Lehto, S.; Rönnemaa, T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: A 7-year follow-up study. Diabetes Care 2006, 29, 329–333. [Google Scholar] [CrossRef]
- Fève, B.; Bastard, J.P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 305–311. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front. Immunol. 2017, 8, 1745. [Google Scholar] [CrossRef]
- West, R.M. Best practice in statistics: The use of log transformation. Ann. Clin. Biochem. 2022, 59, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J. Notes on the use of data transformations. Pract. Assess. Res. Eval. 2002, 8, 6. [Google Scholar] [CrossRef]
- Noor, A.; Rahman, M.U.; Faraz, N.; Samin, K.A.; Ullah, H.; Ali, A. Relationship of Homocysteine with Gender, Blood Pressure, Body Mass Index, Hemoglobin A1c, and the Duration of Diabetes Mellitus Type 2. Cureus 2021, 13, e19211. [Google Scholar] [CrossRef] [PubMed]
- Kotchapetch, H.; Rintra, J.; Sittiprapaporn, P. Association Between HBA1C and Inflammatory Markers: HS-CRP and Homocysteine. In Proceedings of the AU Hybrid International Conference 2024 on “Entrepreneurship & Sustainability in the Digital Era” Under the Theme of “People Centric Knowledge in Intelligence World”, Hua Mak, Thailand, 26 April 2024; pp. 458–464. [Google Scholar]
- Hoogeveen, E.K.; Kostense, P.J.; Jakobs, C.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes: 5-year follow-up of the Hoorn Study. Circulation 2000, 101, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Zulfania Khan, A.; Rehman, S.; Ghaffar, T. Association of homocysteine with body mass index, blood pressure, HbA1c and duration of diabetes in type 2 diabetics. Pak. J. Med. Sci. 2018, 34, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Calzoni, F.; Volpato, S.; Nora, E.D.; Pareschi, P.L.; Zamboni, P.F.; Fellin, R.; Solini, A. Effect of metabolic control on homocysteine levels in type 2 diabetic patients: A 3-year follow-up. J. Intern. Med. 2003, 254, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Kuo, H.T.; Chiu, Y.W.; Hwang, S.J.; Chuang, H.Y.; Chang, J.M.; Chen, H.C.; Lai, Y.H. Correlation of plasma homocysteine level with arterial stiffness and pulse pressure in hemodialysis patients. Atherosclerosis 2005, 182, 121–127. [Google Scholar] [CrossRef]
- Sundström, J.; Sullivan, L.; D’Agostino, R.B.; Jacques, P.F.; Selhub, J.; Rosenberg, I.H.; Wilson, P.W.; Levy, D.; Vasan, R.S. Plasma homocysteine, hypertension incidence, and blood pressure tracking: The Framingham Heart Study. Hypertension 2003, 42, 1100–1105. [Google Scholar] [CrossRef]
- Pettersson-Pablo, P.; Nilsson, T.K.; Breimer, L.H.; Hurtig-Wennlöf, A. Body fat percentage is more strongly associated with biomarkers of low-grade inflammation than traditional cardiometabolic risk factors in healthy young adults—The Lifestyle, Biomarkers, and Atherosclerosis study. Scand. J. Clin. Lab. Investig. 2019, 79, 182–187. [Google Scholar] [CrossRef]
- Garcia-Rubira, J.C.; Cano-Garcia, F.J.; Bullon, B.; Seoane, T.; Villar, P.V.; Cordero, M.D.; Bullon, P. Body fat and metabolic age as indicators of inflammation and cardiovascular risk. Eur. J. Prev. Cardiol. 2018, 25, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Montecucco, F.; Tardif, J.C.; Libby, P.; Camici, G.G. Inflamm-ageing: The role of inflammation in age-dependent cardiovascular disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Navarro, K.M.; Kleinman, M.T.; Mackay, C.E.; Reinhardt, T.E.; Balmes, J.R.; Broyles, G.A.; Ottmar, R.D.; Naher, L.P.; Domitrovich, J.W. Wildland firefighter smoke exposure and risk of lung cancer and cardiovascular disease mortality. Environ. Res. 2019, 173, 462–468. [Google Scholar] [CrossRef]
- Brattström, L.; Wilcken, D.E. Homocysteine and cardiovascular disease: Cause or effect? Am. J. Clin. Nutr. 2000, 72, 315–323. [Google Scholar] [CrossRef]
- Smulders, Y.M.; Blom, H.J. The homocysteine controversy. J. Inherit. Metab. Dis. 2011, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Riddell, L.J.; Chisholm, A.; Williams, S.; Mann, J.I. Dietary strategies for lowering homocysteine concentrations. Am. J. Clin. Nutr. 2000, 71, 1448–1454. [Google Scholar] [CrossRef]
- Malinow, M.R.; Duell, P.B.; Hess, D.L.; Anderson, P.H.; Kruger, W.D.; Phillipson, B.E.; Gluckman, R.A.; Block, P.C.; Upson, B.M. Reduction of plasma homocyst(e)ine levels by breakfast cereal fortified with folic acid in patients with coronary heart disease. N. Engl. J. Med. 1998, 338, 1009–1015. [Google Scholar] [CrossRef]
- Nygård, O.; Refsum, H.; Ueland, P.M.; Vollset, S.E. Major lifestyle determinants of plasma total homocysteine distribution: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 1998, 67, 263–270. [Google Scholar] [CrossRef]
- Moat, S.J.; Lang, D.; McDowell, I.F.; Clarke, Z.L.; Madhavan, A.K.; Lewis, M.J.; Goodfellow, J. Folate, homocysteine, endothelial function and cardiovascular disease. J. Nutr. Biochem. 2004, 15, 64–79. [Google Scholar] [CrossRef]
- Schnell, O.; Amann-Zalan, I.; Jelsovsky, Z.; Moritz, A.; Bermejo, J.L.; Parkin, C.G.; Schweitzer, M.A.; Fisher, L.; Polonsky, W.H. Changes in A1C levels are significantly associated with changes in levels of the cardiovascular risk biomarker hs-CRP: Results from the SteP study. Diabetes Care 2013, 36, 2084–2089. [Google Scholar] [CrossRef]
- Orysiak, J.; Młynarczyk, M.; Piec, R.; Jakubiak, A. Lifestyle and environmental factors may induce airway and systemic inflammation in firefighters. Environ. Sci. Pollut. Res. Int. 2022, 29, 73741–73768. [Google Scholar] [CrossRef] [PubMed]
- Soteriades, E.S.; Smith, D.L.; Tsismenakis, A.J.; Baur, D.M.; Kales, S.N. Cardiovascular disease in US firefighters: A systematic review. Cardiol. Rev. 2011, 19, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Warner, R. Applied Statistics: From Bivariate Through Multivariate Techniques, 3rd ed.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2021. [Google Scholar]
| Resting Cardiovascular Characteristics | |
|---|---|
| Measure (Unit) | Mean ± Standard Deviation |
| Systolic Blood Pressure (mmHg) | 132.2 ± 8.6 |
| Diastolic Blood Pressure (mmHg) | 70.6 ± 9.5 |
| Pulse Pressure (mmHg) | 62.3 ± 5.7 |
| Mean Arterial Pressure (mmHg) | 94.7 ± 8.8 |
| Heart Rate (bpm) | 57.4 ± 7.8 |
| Estimate Stroke Volume (mL) | 115.7 ± 21.1 |
| Peripheral Resistance (PRU) | 0.9 ± 0.2 |
| Pulse Wave Velocity (m/s) | 6.5 ± 0.9 |
| Body Composition Characteristics | |
| Weight (lb) | 220.8 ± 39.6 |
| Height (in) | 70.8 ± 2.8 |
| BMI (kg/m2) | 31.0 ± 5.2 |
| Body Fat (%) | 28.1 ± 7.3 |
| Lean Body Mass (lb) | 156.7 ± 19.4 |
| Total Body Water (lb) | 115.1 ± 14.0 |
| Variable (Units) | Mean | Standard Deviation |
|---|---|---|
| CRP (mg/L) | 1.342 | 0.998 |
| Homocysteine (μmol/L) | 12.698 | 1.083 |
| HOMA-IR | 1.592 | 1.083 |
| HbA1c (%) | 4.645 | 0.505 |
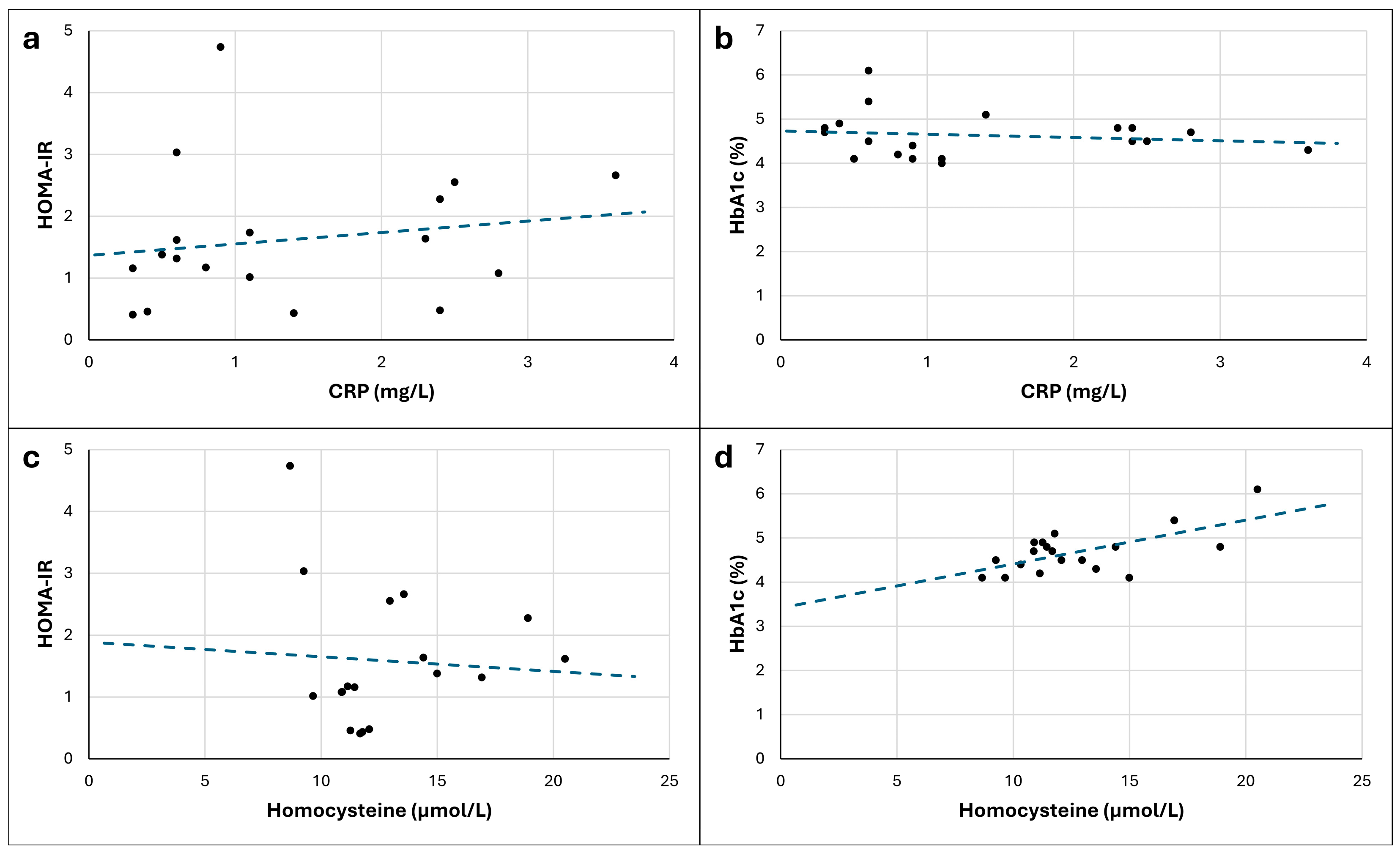
| Variables | Zero-Order Correlations | Partial Correlations | |||
| r | p | rpartial | ppartial | ||
| Non-transformed scores | CRP | ||||
| HOMA-IR | 0.170 | 0.499 | −0.024 | 0.929 | |
| HbA1c | −0.144 | 0.556 | −0.059 | 0.822 | |
| Homocysteine | |||||
| HOMA-IR | −0.069 | 0.787 | −0.351 | 0.183 | |
| HbA1c | 0.643 | 0.003 * | 0.632 | 0.007 * | |
| Variables | Zero-Order Correlations | Partial Correlations | |||
| r | p | rpartial | ppartial | ||
| Transformed scores | CRP | ||||
| HOMA-IR | 0.231 | 0.356 | −0.063 | 0.818 | |
| HbA1c | −0.150 | 0.541 | −0.083 | 0.752 | |
| Homocysteine | |||||
| HOMA-IR | 0.011 | 0.964 | −0.305 | 0.250 | |
| HbA1c | 0.605 | 0.006 * | 0.588 | 0.013 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberther, T.J.; Moore, A.R.; Kohler, A.A.; Holland-Winkler, A.M. Relationship Between Systemic Inflammation and Glycemic Control in Firefighters. J. Funct. Morphol. Kinesiol. 2025, 10, 148. https://doi.org/10.3390/jfmk10020148
Oberther TJ, Moore AR, Kohler AA, Holland-Winkler AM. Relationship Between Systemic Inflammation and Glycemic Control in Firefighters. Journal of Functional Morphology and Kinesiology. 2025; 10(2):148. https://doi.org/10.3390/jfmk10020148
Chicago/Turabian StyleOberther, Tiffany J., Andrew R. Moore, Austin A. Kohler, and A. Maleah Holland-Winkler. 2025. "Relationship Between Systemic Inflammation and Glycemic Control in Firefighters" Journal of Functional Morphology and Kinesiology 10, no. 2: 148. https://doi.org/10.3390/jfmk10020148
APA StyleOberther, T. J., Moore, A. R., Kohler, A. A., & Holland-Winkler, A. M. (2025). Relationship Between Systemic Inflammation and Glycemic Control in Firefighters. Journal of Functional Morphology and Kinesiology, 10(2), 148. https://doi.org/10.3390/jfmk10020148







