Contributions of Medications, Physical and Hydrotherapy Programs in Reducing Endothelial Dysfunction in Hypertensive Patients
Abstract
1. Introduction
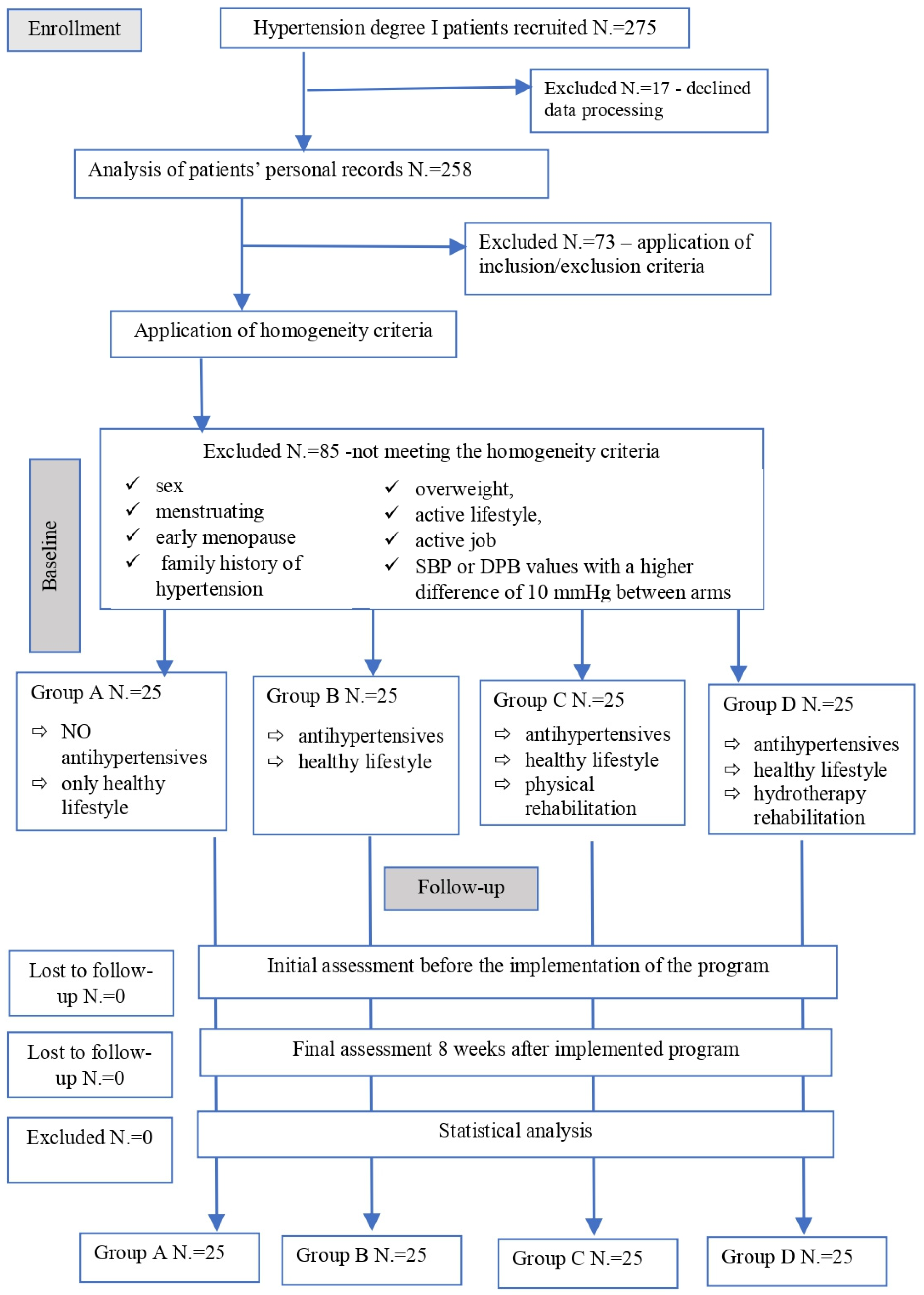
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Lifestyle Change
2.4. Antihypertensive Medication
2.5. Cardiovascular Physical Therapy
- Section 1 was constituted by warm-up exercises in order to prepare the body for effort: 10–15 min of stretching and mobilization of all body segments to activate the body’s circulatory system, without inducing tiredness; the aim of this section is to prevent incidents that may occur during the second part.
- Section 2 is the main part, the exertion for 30 to 45 min in order to reach 75% of their effort capacity; to increase patients’ interest and to avoid boredom, a circuit was built on three main stations, i.e., walking on treadmill, ergo-bike and climbing stairs, as well as other types of physical exercise in between stations that stimulate all body parts, every muscle group and the entire cardiovascular system. This circuit was improved and modified every time it was necessary in order to fulfill all patients’ needs and to obtain the best rehabilitation feedback.
- Section 3 involved recovery after effort with respiratory and relaxation methods in order to remove fatigue. This consisted of a minimum of 15 and up to 20 min of combined techniques between active relaxation and respiratory exercises: slow mobilization of the limbs and trunk in the rhythm of breathing, sometimes with isometric pauses that help release accumulated muscle tension; for the last 10 min, full body relaxation may be induced using different techniques like those of Schultz or Jacobson [38,39].
2.6. Cardiovascular Hydrotherapy Program
- Section 1 consisted of 15–20 min of preparing the patients for effort by using auxiliary means of physical therapy: magnetotherapy with lumbar and cervical applications; four cellular galvanic baths at a temperature of 37–38 °C with negative polarity in the lower limbs and positive in the upper limbs; bath in a pool with warm sodium chloride mineral water; light bath with dry air by infrared irradiation; dry air sauna; and infrared sauna.
- Section 2 was the main part of the exercise: in a pool with regular water, using its resistance and specific floating devices, all body parts were stimulated along with the whole cardiovascular system. A circuit was built, consisting of different stations, i.e., walking, cycling and climbing stairs underwater, combined with other types of physical exercise for at least 30 and up to 40 min.
- Section 3 involved lower intensity practice in order to induce recovery and relaxation after the effort, consisting of 15–20 min of freestyle swimming combined with floating; the finest way to achieve maximal relaxation was by using floating devices for all patients, even for those who proved to have very good swimming skills.
2.7. Parameters Evaluated
- Improving the lipid profile by increasing protective lipid reactions of high-density lipoprotein cholesterol (HDL) and decreasing atherogenic fractions of low-density lipoprotein cholesterol (LDL);
- Improving the platelet level (PLT) to ensure aggregation function and decrease the risks of thrombosis;
- Stimulation of fibrinolysis, fibrinogen (FI) and thrombus destruction;
- Lowering the inflammation markers from blood and urine, interleukin-6 (IL-6), leukocyte (WBC), neutrophils (NEU) and C-reactive protein (CRP);
- Controlling early markers of renal disorders caused by hypertension: serum creatinine (sCr), microalbuminuria (MA) and urinary creatinine (uCr), as well as the urinary albumin/creatinine ratio (uACR).
2.8. Statistical Analysis
3. Results
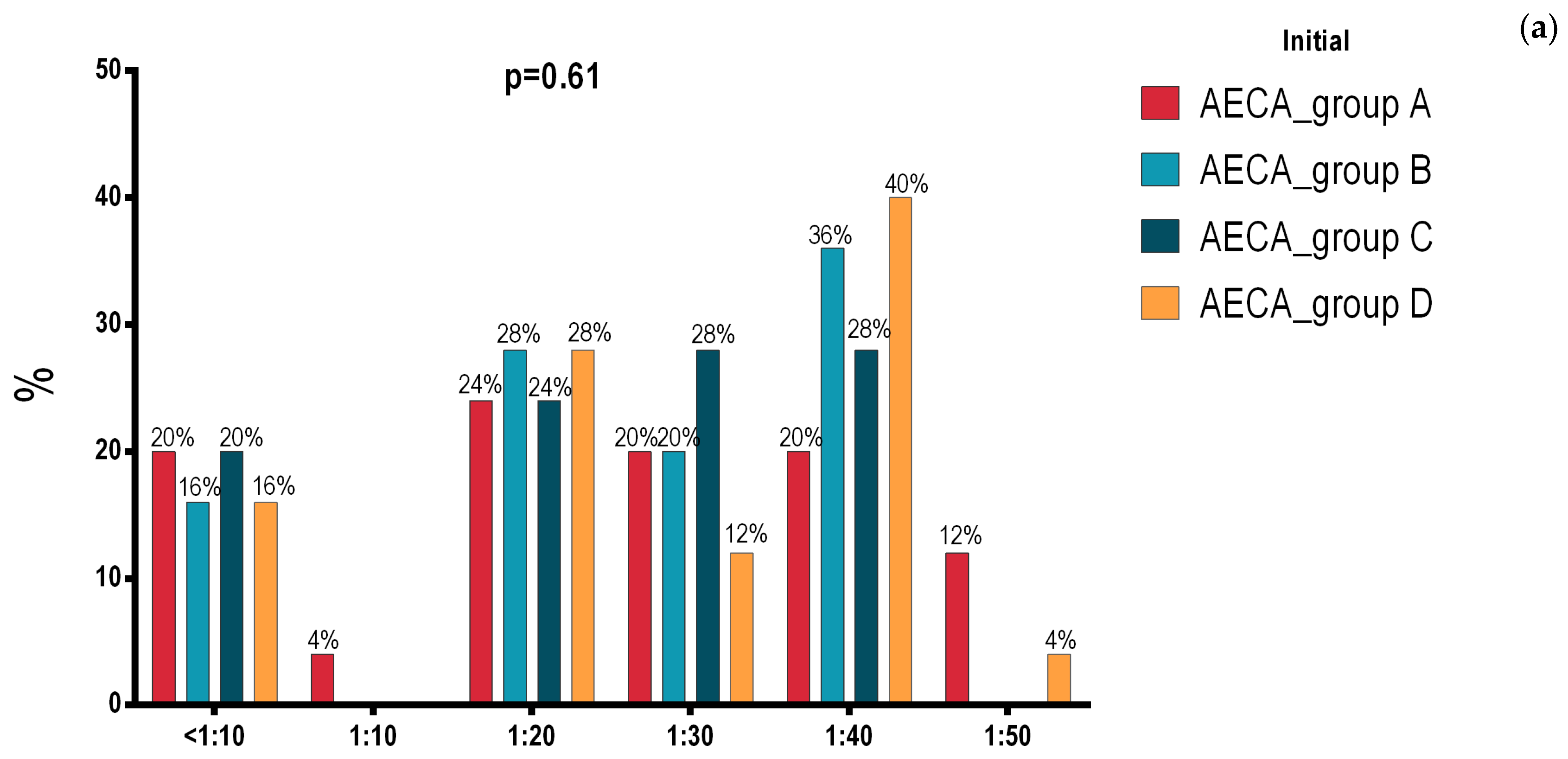
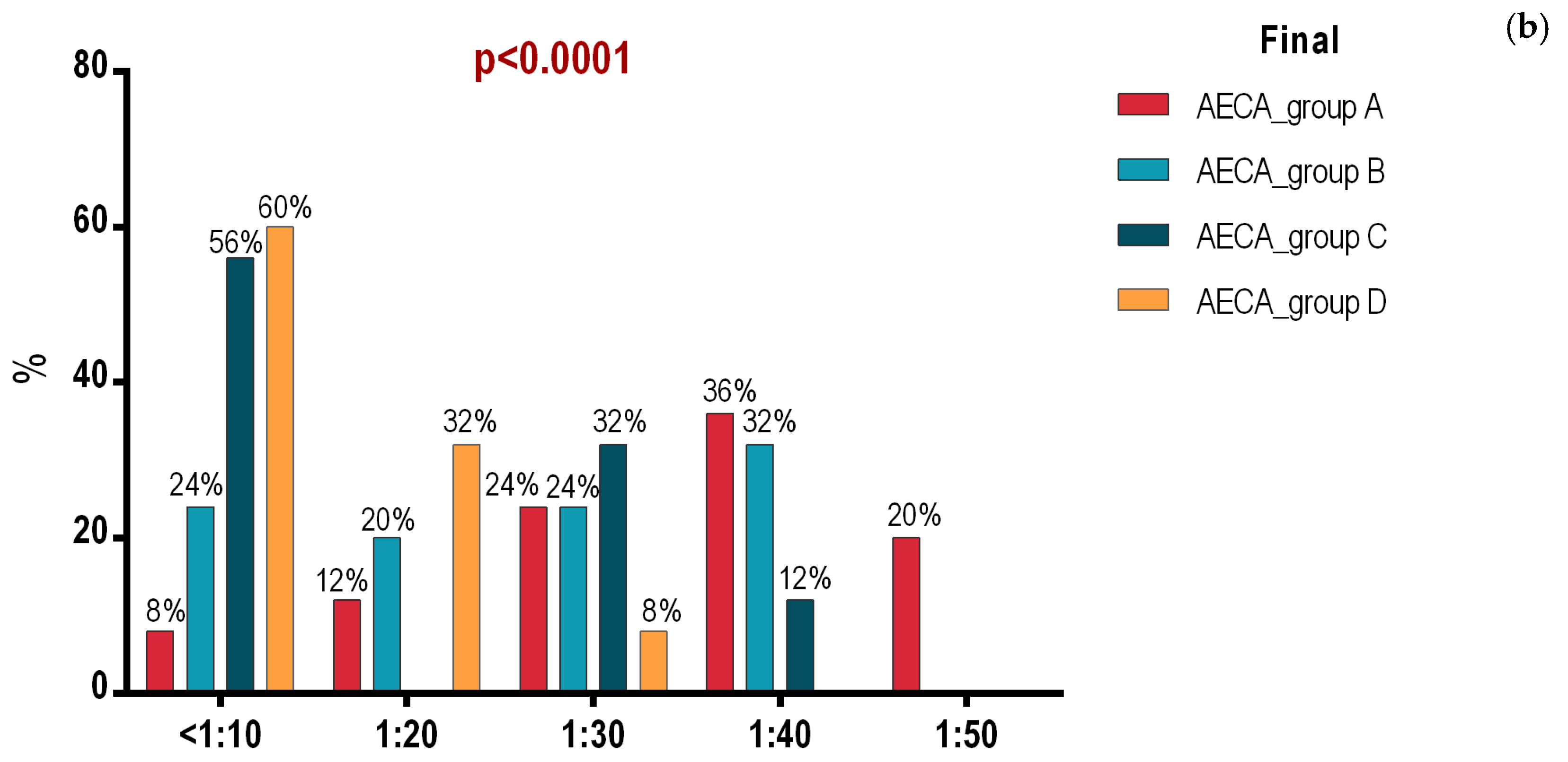
3.1. Initial and Final Intergroup Analysis
3.2. Intragroup Analysis of Initial and Final Assessment in Groups A, B, C and D
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| IL-6 | interleukin-6 |
| ADMA | asymmetric dimethylarginine |
| AECA | anti-endothelial cell antibodies |
| HGB | hemoglobin |
| PLT | platelets |
| WBC | leukocytes |
| NEU | neutrophils |
| HDL | high-density lipoprotein cholesterol |
| LDL | low-density lipoprotein cholesterol |
| CRP | C-reactive protein |
| FI | fibrinogen |
| sCr | serum creatinine |
| MA | microalbuminuria |
| uCr | urinary creatinine |
| uACR | urinary albumin/creatinine ratio |
References
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Word Health Organization (WHO). Hypertension [Internet]. 16 March 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 10 October 2023).
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Kotta, P.A.; Nambi, V. Biomarker guided management of hypertension. Curr. Opin. Nephrol. Hypertens. 2023, 32, 427–433. [Google Scholar] [CrossRef]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E., Jr.; Epstein, S.E. Abnormal endothelium dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Raij, L. Hypertension, endothelium and cardiovascular risk factors. Am. J. Med. 1991, 90, 13S–18S. [Google Scholar] [CrossRef]
- Drexler, H. Endothelial dysfunction: Clinical implications. Prog. Cardiovasc. Dis 1997, 39, 287–324. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Taddei, S.; Salvetti, A. Endothelial dysfunction in essential hypertension: Clinical implications. J. Hypertens. 2002, 20, 1671–1674. [Google Scholar] [CrossRef]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: A randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep. 2020, 10, 7628. [Google Scholar] [CrossRef]
- Craighead, D.A.H.; Heinbockel, T.A.C.; Freeberg, K.A.; Rossman, M.J.; Jackman, R.A.; Jankowski, L.R.; Hamilton, M.N.; Ziemba, B.P.; Reisz, J.A.; D’Alessandro, A.; et al. Time-Efficient Inspiratory Muscle Strength Training Lowers Blood Pressure and Improves Endothelial Function, NO Bioavailability, and Oxidative Stress in Midlife/Older Adults With Above-Normal Blood Pressure. J. Am. Heart Assoc. 2021, 10, e020980. [Google Scholar] [CrossRef]
- Papathanasiou, J.V.; Petrov, I.; Tsekoura, D.; Dionyssiotis, Y.; Ferreira, A.S.; Lopes, A.J.; Ljoka, C.; Foti, C. Does group-based high-intensity aerobic interval training improve the inflammatory status in patients with chronic heart failure? Eur. J. Phys. Rehabil. Med. 2022, 58, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Woolf, E.K.; Terwoord, J.D.; Litwin, N.S.; Vazquez, A.R.; Lee, S.Y.; Ghanem, N.; Michell, K.A.; Smith, B.T.; Grabos, L.E.; Ketelhut, N.B.; et al. Daily blueberry consumption for 12 weeks improves endothelial function in postmenopausal women with above-normal blood pressure through reductions in oxidative stress: A randomized controlled trial. Food Funct. 2023, 14, 2621–2641. [Google Scholar] [CrossRef] [PubMed]
- Scalvini, S.; Olivares, A.; Giardini, A.; Comini, L.; Zanelli, E.; Corica, G.; Genta, T.F. ICF framework in cardiac rehabilitation: A real-life implementation in post-cardiac surgery and chronic heart failure patients. Eur. J. Phys. Rehabil. Med. 2023, 59, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, Ș. Exercise and Hypertension. Adv. Exp. Med. Biol. 2020, 1228, 153–167. [Google Scholar] [CrossRef]
- Saco-Ledo, G.; Valenzuela, P.L.; Ruiz-Hurtado, G.; Ruilope, L.M.; Lucia, A. Exercise Reduces Ambulatory Blood Pressure in Patients With Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e018487. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Rizzoni, D.; Palatini, P. Microcirculation and Physical Exercise in Hypertension. Hypertension 2023, 80, 730–739. [Google Scholar] [CrossRef]
- Palermi, S.; Bragazzi, N.L.; Cular, D.; Ardigò, L.P.; Padulo, J. How chest press-based exercises can alleviate the burden of cardiovascular diseases. Hum. Mov. 2022, 23, 88–98. [Google Scholar] [CrossRef]
- Besson, D.; Sow, A.K.; Fournel, I.; Gouteron, A.; Gudjoncik, A.; Casillas, J.M.; Ornetti, P.; Laroche, D. Impact of eccentric cycling in coronary rehabilitation program: A pragmatic randomized controlled trial versus conventional rehabilitation. Eur. J. Phys. Rehabil. Med, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Scarponi, F.; Zampolini, M.; Zucchella, C.; Bargellesi, S.; Fassio, C.; Pistoia, F.; Bartolo, M. Identifying clinical complexity in patients affected by severe acquired brain injury in neurorehabilitation: A cross sectional survey. Eur. J. Phys. Rehabil. Med. 2019, 55, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Batalik, L.; Dosbaba, F.; Hartman, M.; Konecny, V.; Batalikova, K.; Spinar, J. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur. J. Phys. Rehabil. Med. 2021, 57, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic. Biol. Med. 2017, 109, 4–10. [Google Scholar] [CrossRef]
- Shimokawa, H.; Godo, S. Nitric oxide and endothelium-dependent hyperpolarization mediated by hydrogen peroxide in health and disease. Basic Clin. Pharmacol. Toxicol. 2020, 127, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Khattab, E.; Velidakis, N.; Gkougkoudi, E. The Role of Osteopontin in Atherosclerosis and Its Clinical Manifestations (Atherosclerotic Cardiovascular Diseases)—A Narrative Review. Biomedicines 2023, 11, 3178. [Google Scholar] [CrossRef]
- Vekic, J.; Stromsnes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Gál, R.; Halmosi, R.; Gallyas, F., Jr.; Tschida, M.; Mutirangura, P.; Tóth, K.; Alexy, T.; Czopf, L. Resveratrol and beyond: The Effect of Natural Polyphenols on the Cardiovascular System: A Narrative Review. Biomedicines 2023, 11, 2888. [Google Scholar] [CrossRef]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Yu, L.; Li, G.; He, H.; Lv, Y. Effects of Different Intensities and Durations of Aerobic Exercise on Vascular Endothelial Function in Middle-Aged and Elderly People: A Meta-analysis. Front. Physiol. 2022, 12, 803102. [Google Scholar] [CrossRef]
- Vamvakis, A.; Gkaliagkousi, E.; Lazaridis, A.; Grammatikopoulou, M.G.; Triantafyllou, A.; Nikolaidou, B. Impact of Intensive Lifestyle Treatment (Diet Plus Exercise) on Endothelial and Vascular Function, Arterial Stiffness and Blood Pressure in Stage 1 Hypertension: Results of the HINTreat Randomized Controlled Trial. Nutrients 2020, 12, 1326. [Google Scholar] [CrossRef] [PubMed]
- Morishima, T.; Iemitsu, M.; Ochi, E. Short-term cycling restores endothelial dysfunction after resistance exercise. Scand. J. Med. Sci. Sports 2019, 29, 1115–1120. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Puiu, V. Arterial Hypertesnion. Institutional Clinical Protocol; IMSP AMT Botanica: Chişinău, Moldavia, 2009. [Google Scholar]
- Gahram, I.; Atar, D. European guidelines for the prevention of cardiovascular diseases in medical practice. Eur. J. Prev. Cardiol. Cardiovasc. Recovery 2007, 14, E1–E40. [Google Scholar]
- AIMS. Cardiology. In Vascular Surgery and Cardiac Surgery, 8th ed.; AIMS—Academia Italian Medici Specializzandi: Bari, Italy, 2022. [Google Scholar]
- Kennerley, H. Relaxation scripts. In OCTC Practical Guides; Oxford Cognitive Therapy Centre: Oxford, UK, 2016. [Google Scholar]
- Cappello, B.; Chowdhury, N.; Dorji, G.; Farrington, J.; Khan, T.; Ordunez, P.; Shongwe, S.; Slama, S.; Varghese, C.; Vitoria, M.; et al. Guideline for the Pharmacological Treatment of Hypertension in Adults; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003398-6. [Google Scholar]
- Mushtaq, B.; Khan, A.A. Jacobson Muscle Relaxatation Technique (Jpmr) (20 Min). JOJ Nurse Health Care 2018, 8, JOJNHC.MS.ID.555726. [Google Scholar] [CrossRef]
- Pop, D.; Zdrenghea, D.; Roșu, R.; Gușteu, G.; Mureșan, L.; Cismaru, G. Cardiovascular Recovery; University Medical Iuliu Hațieganu: Cluj-Napoca, Romania, 2016; pp. 24–26. ISBN 978-973-693-721-7. [Google Scholar]
- Cinteza, D.; Marcu, V. Cardiovascular Medical Recovery; Balneră: Bucharest, Romania, 2011; pp. 51–62, 54–59. ISBN 978-606-92826-2-5. [Google Scholar]
- Dumitrașcu, M.; Muntean, C.; Lazarescu, H. Hydrotherapy. Balneo-Res. J. 2012, 3, 22–27. [Google Scholar] [CrossRef]
- Turri-Silva, N.; Vale-Lira, A.; Verboven, K.; Durigan, J.L.Q.; Hansen, H.; Gerson, C., Jr. High-intensity interval training versus progressive high-intensity circuit resistance training on endothelial function and cardiorespiratory fitness in heart failure: A preliminary randomized controlled trial. PLoS ONE 2021, 16, e0257607. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Hambrecht, R. Treatment strategies in endothelial dysfunction: Physical exercise versus pharmacological therapy. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 318–320. [Google Scholar] [CrossRef]
- Enescu, B.D. The Importance of Kinetic Recovery Programs in Cardiovascular Pathology; Universitaria Craiova and Prouniversitaria: Bucharest, Romania, 2014; p. 9. [Google Scholar]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, H.; He, H.; Tang, C. Effects of different training modes on hemodynamics and vascular endothelial function in young obese adults. Sci. Rep. 2025, 15, 12608. [Google Scholar] [CrossRef]
- Wen, Z.; Kim, Y.; Choi, Y. Effects of Exercise Program on Mental, Pulmonary, and Cardiovascular Health of Elderly Men with Acquired Severe Physical Disabilities: A Retrospective Study. Healthcare 2025, 13, 597. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Yin, H.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Exercise training and endothelial function in patients with type 2 diabetes: A meta-analysis. Cardiovasc. Diabetol. 2018, 17, 64. [Google Scholar] [CrossRef]
| Group A | Group B | Group C | Group D | p Value | Sig. dif. | |
|---|---|---|---|---|---|---|
| SBP | 147.2 ± 8.76 | 150 ± 5.90 | 150.9 ± 6.25 | 150.5 ± 6.98 | 0.24 | |
| DBP | 94.12 ± 2.97 | 94.8 ± 2.21 | 94.4 ± 2.48 | 94.24 ± 2.5 | 0.79 | |
| ADMA | 123.0 (97–140) | 115 (91.0–142.0) | 134 (103.5–143.5) | 123 (99–139) | 0.76 | |
| HDL | 53.56 ± 11.80 | 48.96 ± 16.43 | 52.64 ± 13.12 | 56.96 ± 12.74 | 0.19 | |
| LDL | 111.4 ± 20.18 | 101.4 ± 28.85 | 107.3 ± 23.73 | 110.2 ± 18.80 | 0.41 | |
| IL-6 | 2.35 (1.89–3.38) | 2.47 (1.73–4.72) | 3.16 (1.84–4.3) | 2.68 (1.83–4.56) | 0.29 | |
| CRP | 0.25 (0.16–0.41) | 0.31 (0.22–0.47) | 0.36 (0.22–0.46) | 0.34 (0.17–0.44) | 0.2 | |
| WBC | 5780 ± 1088 | 7481 ± 2127 | 6832 ± 8025 | 6325 ± 2028 | 0.005 | A-B |
| NEU | 3170 (2505–3530) | 5151 (2230–6230) | 4250 (3525–5455) | 4770 (3870–5485) | 0.02 | A-B, A-D |
| PLT | 236,000 (193,000–258,500) | 226,000 (173,500–366,000) | 285,000 (233,500–3,515,000) | 280,000 (210,500–340,500) | 0.11 | |
| FI | 2.56 (2.23–2.86) | 3.1 (2.65–3.7) | 3.5 (2.25–3.75) | 3.3 (2.55–4.0) | 0.01 | A-C |
| HGB | 13.15 ± 1.74 | 14.43 ± 1.97 | 15.2 ± 1.54 | 15.07 ± 1.34 | 0.0003 | A-C, A-D |
| sCr | 0.806 ± 0.26 | 0.84 ± 0.25 | 0.84 ± 0.17 | 0.84 ± 0.12 | 0.21 | |
| MA | 5.38 ± 2.59 | 5.42 ± 2.93 | 5.05 ± 2.43 | 5.2 ± 2.41 | 0.95 | |
| uCr | 192.3 ± 75.97 | 251.8 ± 87.72 | 221.5 ± 72.08 | 220 ± 63.78 | 0.056 | |
| uACR | 2.7 (1.5–3.3) | 2.7 (1.85–4.65) | 3.0 (2.25–4.5) | 3.6 (2.3–5.9) | 0.01 | A-D, B-D |
| Group A | Group B | Group C | Group D | p Value | Sig. dif. | |
|---|---|---|---|---|---|---|
| SBP | 150.1 ± 10.24 | 148.2 ± 5.95 | 144.8 ± 4.77 | 144.0 ± 4.43 | 0.005 | A-C, A-D |
| DBP | 95.12 ± 2.63 | 94.2 ± 2.36 | 91.92 ± 2.97 | 90.16 ± 2.52 | <0.0001 | A-C, A-D, B-C, B-D |
| ADMA | 131.0 (98.5–143.0) | 110 (90.50–140.0) | 110.0 (92–121.0) | 109 (85–114) | 0.01 | A-D |
| HDL | 53.32 ± 11.73 | 51.16 ± 15.94) | 68.52 ± 7.43 | 70.96 ± 8.33 | <0.0001 | A-C, A-D, B-C, B-D |
| LDL | 108.7 ± 18.88 | 99.96 ± 28.53 | 88.2 ± 14.40 | 87.76 ± 10.18 | 0.0003 | A-C, A-D |
| IL-6 | 2.15 (1.74–3.38) | 2.35 (1.87–3.89) | 2.14 (1.37–3.19) | 1.6 (1.15–3.40) | 0.08 | |
| CRP | 0.26 (0.19–0.26) | 0.30 (0.20–0.46) | 0.21 (0.11–0.84) | 0.2 (0.10–0.30) | 0.3 | |
| WBC | 5910 (5120–6775) | 6830 (5715–9815) | 8710 (7995–8710) | 7890 (6845–9670) | <0.0001 | A-C, A-D, B-C |
| NEU | 3200 (2500–3870) | 5140 (1970–6215) | 5820 (5000–6665) | 5690 (5230–7230) | <0.0001 | A-C, A-D |
| PLT | 235,000 (191,000–259,000) | 223,000 (172,000–366,500) | 255,000 (184,500–2,965,000) | 255,000 (198,000–297,000) | 0.68 | |
| FI | 2.59 ± 0.56 | 3.14 ± 0.94 | 2.70 ± 0.56 | 2.97 ± 0.59 | 0.11 | |
| HGB | 13.19 ± 1.96 | 14.3 ± 1.98 | 13.86 ± 1.04 | 13.66 ± 0.84 | 0.09 | |
| sCr | 0.79 ± 0.24 | 0.82 ± 0.26 | 0.74 ± 0.16 | 0.74 ± 0.13 | 0.44 | |
| MA | 5.37 ± 2.54 | 5.34 ± 2.95 | 4.52 ± 2.19 | 4.25 ± 1.99 | 0.37 | |
| uCr | 199.4 ± 76.04 | 235.1 ± 80.85 | 201.1 ± 67.78 | 204.2 ± 62.76 | 0.31 | |
| uACR | 2.5 (1.65–3.50) | 2.6 (1.7–4.55) | 2.60 (180–3.80) | 2.50 (1.55–3.95) | 0.89 |
| Initial | Final | p Value | |
|---|---|---|---|
| SBP | 147.2 ± 8.76 | 150.1 ± 10.24 | 0.01 |
| DBP | 94.12 ± 2.97 | 95.12 ± 2.63 | 0.0007 |
| ADMA | 118.6 ± 26.56 | 124.3 ± 30.70 | 0.14 |
| HDL | 53.56 ± 11.80 | 53.32 ± 11.73 | 0.16 |
| LDL | 111.4 ± 20.18 | 108.7 ± 18.88 | 0.002 |
| IL-6 | 2.35 (1.89–3.38) | 2.15 (1.74–3.38) | 0.32 |
| CRP | 0.25 (0.16–0.41) | 0.26 (0.19–0.26) | 0.25 |
| WBC | 5780 (4870–6540) | 5910 (5120–6775) | 0.97 |
| NEU | 3170 (2505–3530) | 3200 (2500–3870) | 0.56 |
| PLT | 237,600 ± 63,984 | 235,560 ± 64,429 | 0.52 |
| FI | 2.60 ± 0.47 | 2.59 ± 0.56 | 0.79 |
| HGB | 13.15 ± 1.74 | 13.19 ± 1.96 | 0.88 |
| sCr | 0.80 ± 0.26 | 0.79 ± 0.24 | 0.34 |
| MA | 5.381 ± 2.59 | 5.37 ± 2.54 | 0.95 |
| uCr | 192.3 ± 75.97 | 199.4 ± 76.04 | 0.28 |
| uACR | 2.7 (1.5–3.3) | 2.5 (1.65–3.50) | 0.34 |
| Initial | Final | p Value | |
|---|---|---|---|
| SBP | 150 ± 5.90 | 148.2 ± 5.95 | <0.0001 |
| DBP | 94.8 ± 2.21 | 94.2 ± 2.36 | 0.002 |
| ADMA | 115 (91–142) | 110 (90.50–140.0) | 0.006 |
| HDL | 48.96 ± 16.43 | 51.16 ± 15.94) | <0.0001 |
| LDL | 101.4 ± 28.85 | 99.96 ± 28.53 | < 0.0001 |
| IL-6 | 2.47 (1.73–4.72) | 2.35 (1.87–3.89) | 0.04 |
| CRP | 0.31 (0.22–0.47) | 0.30 (0.20–0.46) | <0.0001 |
| WBC | 6850 (5765–9835) | 6830 (5715–9815) | 0.02 |
| NEU | 5151 (2230–6230) | 5140 (1970–6215) | <0.0001 |
| PLT | 226,000 (173,500–366,000) | 223,000 (172,000–366,500) | 0.0005 |
| FI | 3.11 ± 0.74 | 3.14 ± 0.94 | 0.77 |
| HGB | 14.43 ± 1.97 | 14.3 ± 1.98 | 0.0002 |
| sCr | 0.84 ± 0.25 | 0.82 ± 0.26 | 0.04 |
| MA | 5.42 ± 2.93 | 5.34 ± 2.95 | 0.11 |
| uCr | 251.8 ± 87.72 | 235.1 ± 80.85 | 0.05 |
| uACR | 2.7 (1.85–4.65) | 2.6 (1.70–4.55) | 0.0001 |
| Initial | Final | p Value | |
|---|---|---|---|
| SBP | 150.9 ± 6.25 | 144.8 ± 4.77 | <0.0001 |
| DBP | 95 (92–96.5) | 92 (90–95) | <0.0001 |
| ADMA | 125.8 ± 25.05 | 104.9 ± 19.84 | 0.001 |
| HDL | 52.64 ± 13.12 | 68.52 ± 7.43 | <0.0001 |
| LDL | 107.3 ± 23.73 | 88.2 ± 14.40 | <0.0001 |
| IL-6 | 3.19 ± 1.56 | 2.415 ± 1.19 | <0.0001 |
| CRP | 0.36 (0.22–0.46) | 0.21 (0.11–0.84) | <0.0001 |
| WBC | 6820 ± 8025 | 8710 ± 10250 | <0.0001 |
| NEU | 4250 ± 5455 | 5820 ± 6665 | <0.0001 |
| PLT | 289,320 ± 70,871 | 251,760 ± 76,739 | <0.0001 |
| FI | 3.5 (2.25–3.75) | 2.8 (2.1–3.2) | <0.0001 |
| HGB | 15.2 ± 1.54 | 13.86 ± 1.04 | <0.0001 |
| sCr | 0.84 ± 0.17 | 0.74 ± 0.16 | <0.0001 |
| MA | 5.05 ± 2.43 | 4.52 ± 2.19 | <0.0001 |
| uCr | 221.5 ± 72.08 | 201.1 ± 67.78 | <0.0001 |
| uACR | 3.56 ± 1.94 | 2.88 ± 1.56 | <0.0001 |
| Initial | Final | p Value | |
|---|---|---|---|
| SBP | 152 (143–157.5) | 143 (140.5–147.0) | <0.0001 |
| DBP | 94.24 ± 2.5 | 90.16 ± 2.52 | <0.0001 |
| ADMA | 123 (99–139) | 109 (85–114) | <0.0001 |
| HDL | 56.96 ± 12.74 | 70.96 ± 8.33 | <0.0001 |
| LDL | 110.2 ± 18.80 | 87.76 ± 10.18 | <0.0001 |
| IL-6 | 2.68 (1.83–4.56) | 1.6 (1.15–3.40) | <0.0001 |
| CRP | 0.34 (0.17–0.44) | 0.2 (0.10–0.30) | <0.0001 |
| WBC | 6325 ± 2028 | 8160 ± 1632 | <0.0001 |
| NEU | 4770 (3870–5485) | 5690 (5230–7230) | <0.0001 |
| PLT | 274,080 ± 79,292 | 250,600 ± 67,775 | 0.001 |
| FI | 3.26 ± 0.75 | 2.97 ± 0.59 | <0.0001 |
| HGB | 15.07 ± 1.34 | 13.66 ± 0.84 | <0.0001 |
| sCr | 0.84 ± 0.12 | 0.74 ± 0.13 | <0.0001 |
| MA | 4.1 (3.2–7.57) | 3.3 (2.65–6.4) | <0.0001 |
| uCr | 220 ± 63.78 | 204.2 ± 62.76 | <0.0001 |
| uACR | 4.08 ± 2.09 | 2.85 ± 1.53 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rad Bodan, R.C.; Dușe, A.O.; Papp, E.G.; Melinte, R.M.; Andor, M. Contributions of Medications, Physical and Hydrotherapy Programs in Reducing Endothelial Dysfunction in Hypertensive Patients. J. Funct. Morphol. Kinesiol. 2025, 10, 150. https://doi.org/10.3390/jfmk10020150
Rad Bodan RC, Dușe AO, Papp EG, Melinte RM, Andor M. Contributions of Medications, Physical and Hydrotherapy Programs in Reducing Endothelial Dysfunction in Hypertensive Patients. Journal of Functional Morphology and Kinesiology. 2025; 10(2):150. https://doi.org/10.3390/jfmk10020150
Chicago/Turabian StyleRad Bodan, Roxana Cristina, Adina Octavia Dușe, Eniko Gabriela Papp, Răzvan Marian Melinte, and Minodora Andor. 2025. "Contributions of Medications, Physical and Hydrotherapy Programs in Reducing Endothelial Dysfunction in Hypertensive Patients" Journal of Functional Morphology and Kinesiology 10, no. 2: 150. https://doi.org/10.3390/jfmk10020150
APA StyleRad Bodan, R. C., Dușe, A. O., Papp, E. G., Melinte, R. M., & Andor, M. (2025). Contributions of Medications, Physical and Hydrotherapy Programs in Reducing Endothelial Dysfunction in Hypertensive Patients. Journal of Functional Morphology and Kinesiology, 10(2), 150. https://doi.org/10.3390/jfmk10020150







