The Influence of Transcranial Direct Current Stimulation on Shooting Performance in Elite Deaflympic Athletes: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- ID 1—37-year-old female with 14 years of training experience. She won the gold medal in the 10-m air rifle at the 2014 European Deaf Shooting Championships.
- ID 2—42-year-old female with 18 years of training experience. She won bronze medals at the 2009 and 2013–Deaflympics, 2015—European Deaf Shooting Championships (2× bronze medals and 2× silver medals), a silver medal at the 2016 World Deaf Shooting Championships, and a silver medal in the mixed 10-m air rifle at the 2019 European Deaf Shooting Championships.
- ID 3—26-year-old man with 12 years of training experience. He won the silver medal in the 10-m air rifle in the 2019 International Competitions “Istvan Poljanac”.
- ID 4—23-year-old man with 12 years of training experience. He won the silver medal in the mixed 10-m air rifle at the 2019 European Deaf Shooting Championships.
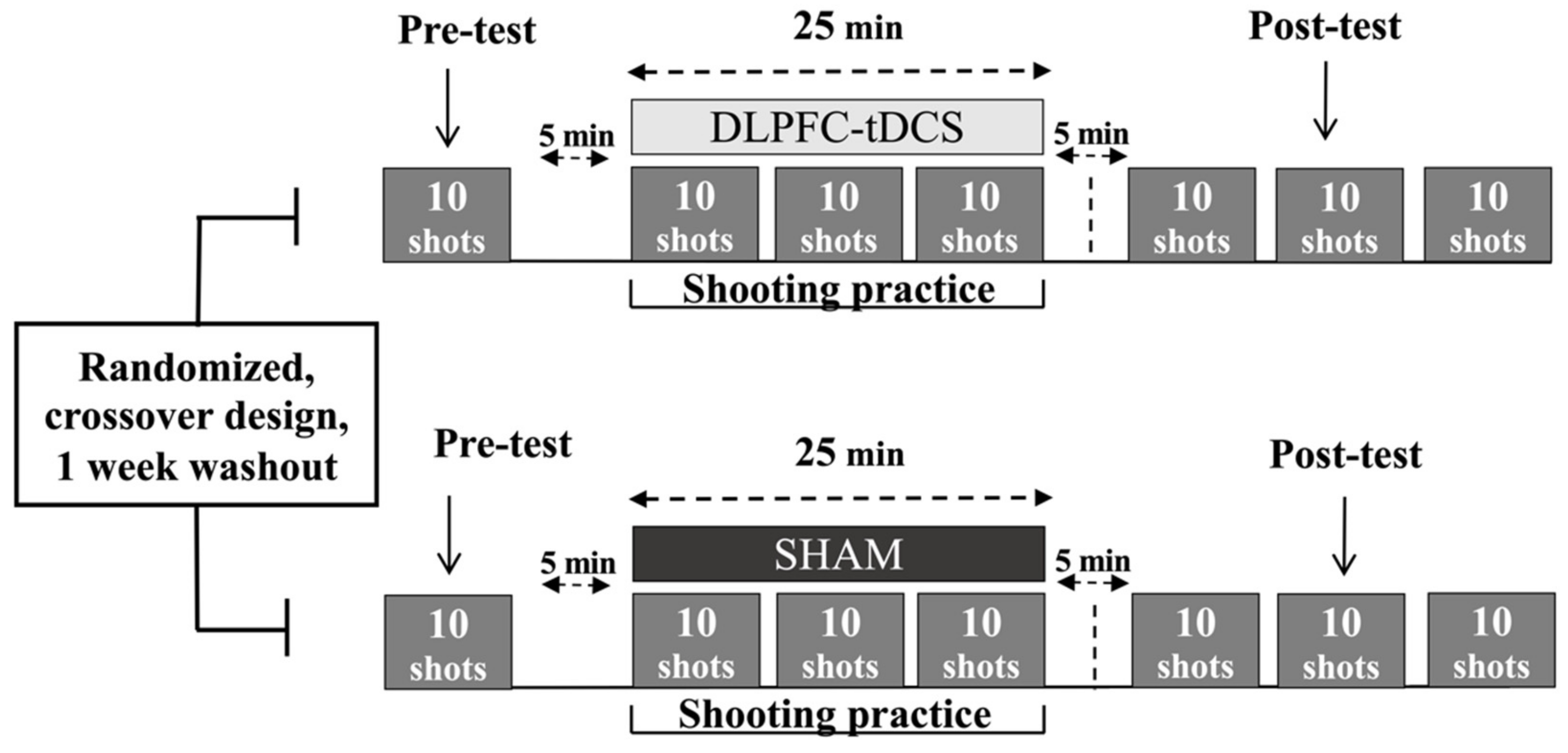
2.2. Experimental Design
2.3. DLPFC-tDCS
2.4. Practice Sessions
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Group Level Observations
3.2. Individual Data
- ID 1 scored more points in the DLPFC-tDCS condition on Days 2 and 3. However, the 3-Day total points score was slightly higher in the SHAM condition (708 vs. 707.4 points). Similarly, the total endpoint error was lower during the DLPFC-tDCS condition on Days 2 and 3. However, the total endpoint error was lower in the SHAM condition (5.5 vs. 5.6 mm).
- ID 2 scored more points in the SHAM condition on Days 1 and 3. Moreover, the 3-Day total points score was slightly higher in the SHAM condition (705.8 vs. 704.1 points). Similarly, the total endpoint error was lower during the SHAM condition on Days 1 and 3. However, the total endpoint error was lower in the SHAM condition (5.6 vs. 5.9 mm).
- ID 3 scored more points in the SHAM condition on Days 2 and 3. Moreover, the 3-Day total points score was slightly higher in the SHAM condition (682.9 vs. 679.3 points). Similarly, the total endpoint error was lower during the SHAM condition on Days 2 and 3. However, the total endpoint error was lower in the SHAM condition (8 vs. 8.1 mm).
- ID 4 scored more points in the DLPFC-tDCS condition on Days 2 and 3. In addition, the 3-Day total points score was slightly higher in the DLPFC-tDCS condition (716.6 vs. 713.6 points). Similarly, the total endpoint error was lower during the DLPFC-tDCS condition on Days 2 and 3. However, the total endpoint error was lower in the DLPFC-tDCS condition (4.6 vs. 5 mm).
4. Discussion
4.1. Influence of DLPFC-tDCS on Motor Skill and Learning in Rifle Shooting
4.2. Possible Factors Responsible for Inability of DLPFC-tDCS to Improve Shooting Performance
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buch, E.R.; Santarnecchi, E.; Antal, A.; Born, J.; Celnik, P.A.; Classen, J.; Gerloff, C.; Hallett, M.; Hummel, F.C.; Nitsche, M.A.; et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin. Neurophysiol. 2017, 128, 589–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitsche, M.A.; Paulus, W. Transcranial Direct Current Stimulation—Update 2011. Restor. Neurol. Neurosci. 2011, 29, 463–492. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.K.; de Albuquerque, L.L.; Pantovic, M.; Fischer, K.M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. Cerebellar Transcranial Direct Current Stimulation Enhances Motor Learning in a Complex Overhand Throwing Task. Cerebellum 2019, 18, 813–816. [Google Scholar] [CrossRef]
- Cantarero, G.; Spampinato, D.; Reis, J.; Ajagbe, L.; Thompson, T.; Kulkarni, K.; Celnik, P. Cerebellar Direct Current Stimulation Enhances on-Line Motor Skill Acquisition through an Effect on Accuracy. J. Neurosci. 2015, 35, 3285–3290. [Google Scholar] [CrossRef]
- Celnik, P. Understanding and Modulating Motor Learning with Cerebellar Stimulation. Cerebellum 2015, 14, 171–174. [Google Scholar] [CrossRef]
- Grimaldi, G.; Argyropoulos, G.; Boehringer, A.; Celnik, P.; Edwards, M.J.; Ferrucci, R.; Galea, J.M.; Groiss, S.J.; Hiraoka, K.; Kassavetis, P.; et al. Non-invasive Cerebellar Stimulation—A Consensus Paper. Cerebellum 2014, 13, 121–138. [Google Scholar] [CrossRef]
- Oldrati, V.; Schutter, D.J.L.G. Targeting the Human Cerebellum with Transcranial Direct Current Stimulation to Modulate Behavior: A Meta-Analysis. Cerebellum 2018, 17, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Van Dun, K.; Bodranghien, F.C.A.A.; Mariën, P.; Manto, M.U. tDCS of the Cerebellum: Where Do We Stand in 2016? Technical Issues and Critical Review of the Literature. Front. Hum. Neurosci. 2016, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Grospretre, S.; Grandperrin, Y.; Nicolier, M.; Gimenez, P.; Vidal, C.; Tio, G.; Haffen, E.; Bennabi, D. Effect of Transcranial Direct Current Stimulation on the Psychomotor, Cognitive, and Motor Performances of Power Athletes. Sci. Rep. 2021, 11, 9731. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Zanto, T.P.; Anguera, J.A.; Lin, Y.-Y.; Gazzaley, A. Delayed enhancement of multitasking performance: Effects of anodal transcranial direct current stimulation on the prefrontal cortex. Cortex 2015, 69, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamali, A.-M.; Nami, M.; Yahyavi, S.-S.; Saadi, Z.K.; Mohammadi, A. Transcranial Direct Current Stimulation to Assist Experienced Pistol Shooters in Gaining Even-Better Performance Scores. Cerebellum 2019, 18, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.; Marinho, V.; Magalhães, F.; Carvalho, V.; Fernandes, T.; Ayres, M.; Crespo, E.; Velasques, B.; Ribeiro, P.; Cagy, M.; et al. Unskilled shooters improve both accuracy and grouping shot having as reference skilled shooters cortical area: An EEG and tDCS study. Physiol. Behav. 2020, 224, 113036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Yeung, A.Y.; Poolton, J.M.; Lee, T.M.; Leung, G.K.; Masters, R.S. Cathodal Transcranial Direct Current Stimulation over Left Dorsolateral Prefrontal Cortex Area Promotes Implicit Motor Learning in a Golf Putting Task. Brain Stimul. 2015, 8, 784–786. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, J.; Kim, S.; Yoon, B. Noninvasive Brain Stimulation over M1 and Dlpfc Cortex Enhances the Learning of Bimanual Isometric Force Control. Hum. Mov. Sci. 2019, 66, 73–83. [Google Scholar] [CrossRef]
- Hupfeld, K.; Ketcham, C.J.; Schneider, H.D. Transcranial direct current stimulation (tDCS) to the supplementary motor area (SMA) influences performance on motor tasks. Exp. Brain Res. 2017, 235, 851–859. [Google Scholar] [CrossRef]
- Vollmann, H.; Conde, V.; Sewerin, S.; Taubert, M.; Sehm, B.; Witte, O.W.; Villringer, A.; Ragert, P. Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul. 2013, 6, 101–107. [Google Scholar] [CrossRef]
- Reis, J.; Fischer, J.T.; Prichard, G.; Weiller, C.; Cohen, L.G.; Fritsch, B. Time-but Not Sleep-Dependent Consolidation of Tdcs-Enhanced Visuomotor Skills. Cereb. Cortex 2015, 25, 109–117. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef] [Green Version]
- Vancleef, K.; Meesen, R.; Swinnen, S.P.; Fujiyama, H. tDCS over left M1 or DLPFC does not improve learning of a bimanual coordination task. Sci. Rep. 2016, 6, 35739. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, L.L.; Fischer, K.M.; Pauls, A.L.; Pantovic, M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. An acute application of transcranial random noise stimulation does not enhance motor skill acquisition or retention in a golf putting task. Hum. Mov. Sci. 2019, 66, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Meek, A.W.; Greenwell, D.; Poston, B.; Riley, Z.A. Anodal tDCS accelerates on-line learning of dart throwing. Neurosci. Lett. 2021, 764, 136211. [Google Scholar] [CrossRef]
- Buttkus, F.; Baur, V.; Jabusch, H.C.; de la Cruz Gomez-Pellin, M.; Paulus, W.; Nitsche, M.A.; Altenmuller, E. Single-Session Tdcs-Supported Retraining Does Not Improve Fine Motor Control in Musician’s Dystonia. Restor. Neurol. Neurosci. 2011, 29, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lattari, E.; Andrade, M.L.; Filho, A.S.; Moura, A.M.; Neto, G.M.; Silva, J.G.; Rocha, N.B.; Yuan, T.F.; Arias-Carrion, O.; Machado, S. Can Transcranial Direct Current Stimulation Improve the Resistance Strength and Decrease the Rating Perceived Scale in Recreational Weight-Training Experience? J. Strength Cond. Res. 2016, 30, 3381–3387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattari, E.; de Oliveira, B.S.; Oliveira, B.R.R.; de Mello Pedreiro, R.C. Effects of transcranial direct current stimulation on time limit and ratings of perceived exertion in physically active women. Neurosci. Lett. 2018, 662, 12–16. [Google Scholar] [CrossRef]
- Alix-Fages, C.; García-Ramos, A.; Calderón-Nadal, G.; Colomer-Poveda, D.; Romero-Arenas, S.; Fernández-Del-Olmo, M.; Márquez, G. Anodal transcranial direct current stimulation enhances strength training volume but not the force–velocity profile. Eur. J. Appl. Physiol. 2020, 120, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Beam, W.; Borckardt, J.J.; Reeves, S.T.; George, M.S. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009, 2, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, N.; Lin, Y.; Chen, R.; Zhang, J. Hemispheric Differences in Functional Interactions Between the Dorsal Lateral Prefrontal Cortex and Ipsilateral Motor Cortex. Front. Hum. Neurosci. 2020, 14, 202. [Google Scholar] [CrossRef]
- Kantak, S.S.; Sullivan, K.J.; E Fisher, B.; Knowlton, B.J.; Winstein, C.J. Neural substrates of motor memory consolidation depend on practice structure. Nat. Neurosci. 2010, 13, 923–925. [Google Scholar] [CrossRef]
- Lima de Albuquerque, L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. An Acute Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Performance in Parkinson’s Disease. Brain Sci. 2020, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Poston, B.; Van Gemmert, A.W.; Sharma, S.; Chakrabarti, S.; Zavaremi, S.H.; Stelmach, G. Movement trajectory smoothness is not associated with the endpoint accuracy of rapid multi-joint arm movements in young and older adults. Acta Psychol. 2013, 143, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljubisavljevic, M.R.; Oommen, J.; Filipovic, S.; Bjekic, J.; Szolics, M.; Nagelkerke, N. Effects of tDCS of Dorsolateral Prefrontal Cortex on Dual-Task Performance Involving Manual Dexterity and Cognitive Task in Healthy Older Adults. Front. Aging Neurosci. 2019, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, L.L.; Pantovic, M.; Clingo, M.G.; Fischer, K.M.; Jalene, S.; Landers, M.R.; Mari, Z.; Poston, B. Long-Term Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Learning in Parkinson’s Disease. Cerebellum 2021, 21, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Cosentino, G.; Brighina, F.; Pozzi, N.G.; Sandrini, G.; Fierro, B.; Savettieri, G.; D’Amelio, M.; Pacchetti, C. Transcranial direct current stimulation for treatment of freezing of gait: A cross-over study. Mov. Disord. 2014, 29, 1064–1069. [Google Scholar] [CrossRef]
- Shibata, T.; Urata, A.; Kawahara, K.; Furuya, K.; Ishikuro, K.; Hattori, N.; Kuroda, S. Therapeutic Effects of Diagonal-Transcranial Direct Current Stimulation on Functional Recovery in Acute Stroke: A Pilot Study. J. Stroke Cerebrovasc. Dis. 2020, 29, 105107. [Google Scholar] [CrossRef]
- Miterko, L.N.; Baker, K.B.; Beckinghausen, J.; Bradnam, L.V.; Cheng, M.Y.; Cooperrider, J.; DeLong, M.R.; Gornati, S.V.; Hallett, M.; Heck, D.H.; et al. Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum 2019, 18, 1064–1097. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Zoghi, M.; Jaberzadeh, S. Biological and anatomical factors influencing interindividual variability to noninvasive brain stimulation of the primary motor cortex: A systematic review and meta-analysis. Rev. Neurosci. 2018, 29, 199–222. [Google Scholar] [CrossRef]
- Li, L.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Labruna, L.; Jamil, A.; Fresnoza, S.; Batsikadze, G.; Kuo, M.-F.; Vanderschelden, B.; Ivry, R.B.; Nitsche, M.A. Efficacy of Anodal Transcranial Direct Current Stimulation is Related to Sensitivity to Transcranial Magnetic Stimulation. Brain Stimul. 2016, 9, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in Response to Transcranial Direct Current Stimulation of the Motor Cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, V.; Cheeran, B.; Fernández-Del-Olmo, M. Relationship Between Non-invasive Brain Stimulation-induced Plasticity and Capacity for Motor Learning. Brain Stimul. 2015, 8, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; McGlory, C.; Gibala, M.J.; Phillips, S.M. Investigating human skeletal muscle physiology with unilateral exercise models: When one limb is more powerful than two. Appl. Physiol. Nutr. Metab. 2017, 42, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Points | Endpoint Error (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tDCS | SHAM | tDCS | SHAM | |||||||||
| Subject | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 |
| ID 1 | 699.7 | 711.7 | 710.8 | 710.2 | 703.2 | 710.6 | 6.33 | 5.13 | 5.23 | 5.28 | 5.98 | 5.24 |
| ID 2 | 701.6 | 705.7 | 705.1 | 707.4 | 704.4 | 705.6 | 6.14 | 5.73 | 5.79 | 5.55 | 5.86 | 5.37 |
| ID 3 | 685 | 680.6 | 672.2 | 683.4 | 684.4 | 681 | 7.8 | 8.05 | 8.55 | 7.96 | 7.9 | 8.28 |
| ID 4 | 712.7 | 719.2 | 717.9 | 714.1 | 713.3 | 713.5 | 5.03 | 4.38 | 4.51 | 4.88 | 4.97 | 5.01 |
| Average | 699.8 | 704.3 | 701.5 | 703.8 | 701.3 | 702.7 | 6.3 | 5.8 | 6.0 | 5.9 | 6.2 | 6.0 |
| Total Points | Total Endpoint Error (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Confidence Interval | Confidence Interval | ||||||||||
| Subject | Condition | Mean | SD | CV | LL 95% | HL 95% | Mean | SD | CV | LL 95% | HL 95% |
| ID 1 | tDCS | 707.4 | 6.7 | 0.9 | 690.8 | 724.0 | 5.6 | 0.7 | 12 | 3.9 | 7.2 |
| SHAM | 708.0 | 4.2 | 0.6 | 697.7 | 718.3 | 5.5 | 0.4 | 7.6 | 4.5 | 6.5 | |
| ID 2 | tDCS | 704.1 | 2.2 | 0.3 | 698.6 | 709.6 | 5.9 | 0.2 | 3.8 | 5.3 | 6.4 |
| SHAM | 705.8 | 1.5 | 0.2 | 702.0 | 709.6 | 5.6 | 0.2 | 4.4 | 5 | 6.2 | |
| ID 3 | tDCS | 679.3 | 6.5 | 1 | 663.1 | 695.4 | 8.1 | 0.4 | 4.7 | 7.2 | 9.1 |
| SHAM | 682.9 | 1.7 | 0.3 | 678.6 | 687.3 | 8 | 0.2 | 6.8 | 7.5 | 8.6 | |
| ID 4 | tDCS | 716.6 | 3.4 | 0.5 | 708.1 | 725.1 | 4.6 | 0.3 | 7.4 | 3.8 | 5.5 |
| SHAM | 713.6 | 0.4 | 0.1 | 712.6 | 714.7 | 5 | 0.1 | 1.3 | 4.8 | 5.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantovic, M.; Macak, D.; Cokorilo, N.; Moonie, S.; Riley, Z.A.; Madic, D.M.; Poston, B. The Influence of Transcranial Direct Current Stimulation on Shooting Performance in Elite Deaflympic Athletes: A Case Series. J. Funct. Morphol. Kinesiol. 2022, 7, 42. https://doi.org/10.3390/jfmk7020042
Pantovic M, Macak D, Cokorilo N, Moonie S, Riley ZA, Madic DM, Poston B. The Influence of Transcranial Direct Current Stimulation on Shooting Performance in Elite Deaflympic Athletes: A Case Series. Journal of Functional Morphology and Kinesiology. 2022; 7(2):42. https://doi.org/10.3390/jfmk7020042
Chicago/Turabian StylePantovic, Milan, Drazenka Macak, Nebojsa Cokorilo, Sheniz Moonie, Zachary A. Riley, Dejan M. Madic, and Brach Poston. 2022. "The Influence of Transcranial Direct Current Stimulation on Shooting Performance in Elite Deaflympic Athletes: A Case Series" Journal of Functional Morphology and Kinesiology 7, no. 2: 42. https://doi.org/10.3390/jfmk7020042
APA StylePantovic, M., Macak, D., Cokorilo, N., Moonie, S., Riley, Z. A., Madic, D. M., & Poston, B. (2022). The Influence of Transcranial Direct Current Stimulation on Shooting Performance in Elite Deaflympic Athletes: A Case Series. Journal of Functional Morphology and Kinesiology, 7(2), 42. https://doi.org/10.3390/jfmk7020042







