Reliability of a Smooth Pursuit Eye-Tracking System (EyeGuide Focus) in Healthy Adolescents and Adults
Abstract
:1. Introduction
2. Materials and Methods
3. Results
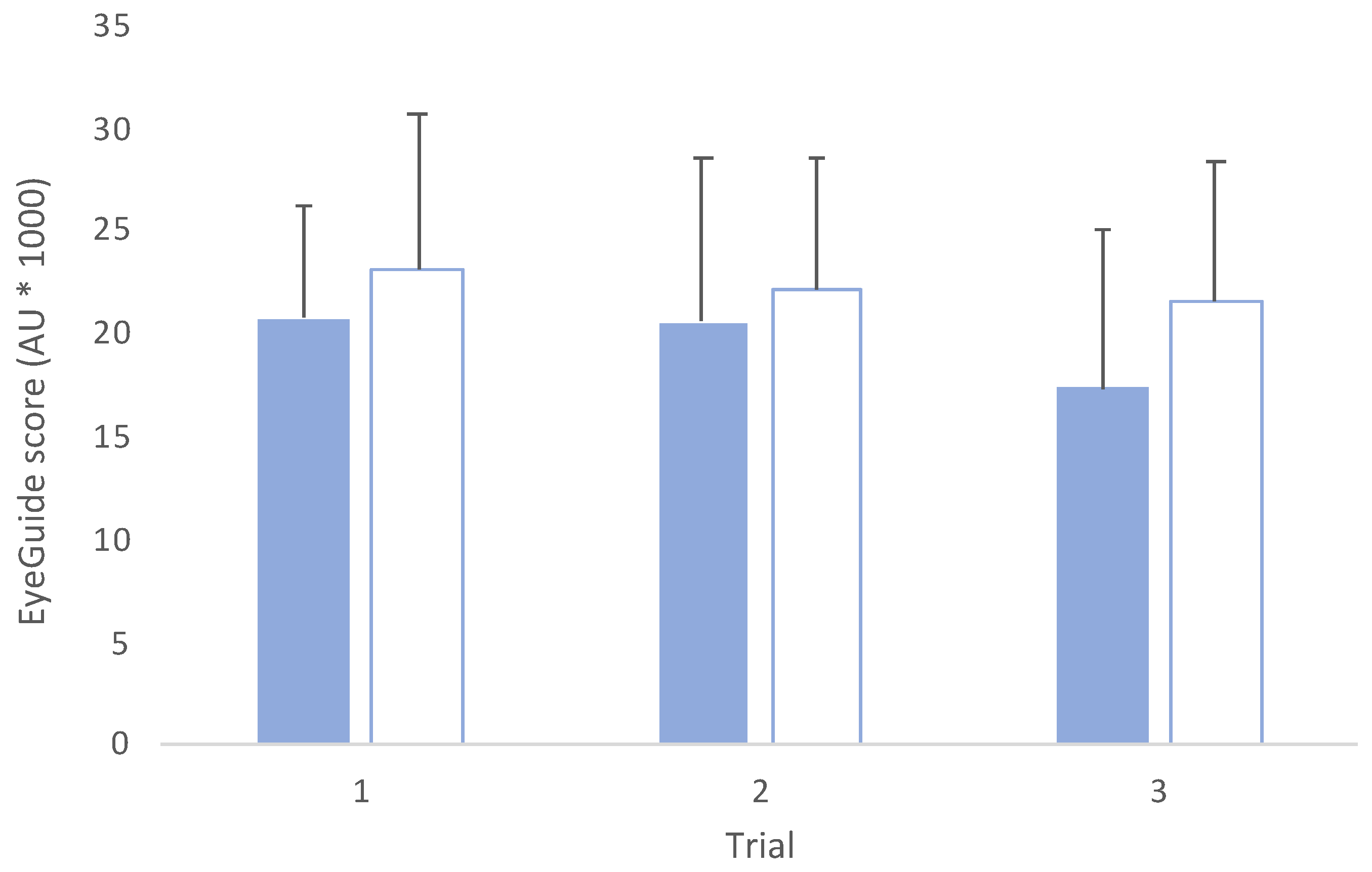
3.1. Test–retest Reliability Analyses
3.2. Comparisons across Trials and Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, A.; Ueda, K. Contribution of eye-tracking to study cognitive impairments among clinical populations. Front. Psychol. 2021, 12, 590986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Z.; Gu, Y.; Liu, H.; Wang, P. The effectiveness of eye tracking in the diagnosis of cognitive disorders: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0254059. [Google Scholar] [CrossRef] [PubMed]
- Snegireva, N.; Derman, W.; Patricios, J.; Welman, K.E. Eye tracking technology in sports-related concussion: A systematic review and meta-analysis. Physiol. Meas. 2018, 39, 12TR01. [Google Scholar] [CrossRef] [PubMed]
- Harmon, K.G.; Clugston, J.R.; Dec, K.; Hainline, B.; Herring, S.; Kane, S.F.; Kontos, A.P.; Leddy, J.J.; McCrea, M.; Poddar, S.K. American Medical Society for Sports Medicine position statement on concussion in sport. Br. J. Sport. Med. 2019, 53, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Ferdinand Pennock, K.; McKenzie, B.; McClemont Steacy, L.; Mainwaring, L. Under-reporting of sport-related concussions by adolescent athletes: A systematic review. Int. Rev. Sport. Exerc. Psychol. 2020, in press. [Google Scholar] [CrossRef]
- Pearce, A.J.; Young, J.A.; Parrington, L.; Aimers, N. Do as I say: Contradicting beliefs and attitudes towards sports concussion in Australia. J. Sport. Sci. 2017, 35, 1911–1919. [Google Scholar] [CrossRef]
- AFL Players’ Association Limited. Insights and Impact Report, 1st ed.; AFL Players’ Association Limited: Melbourne, Australia, 2022. [Google Scholar]
- Pearce, A.J.; Hoy, K.; Rogers, M.A.; Corp, D.T.; Davies, C.B.; Maller, J.J.; Fitzgerald, P.B. Acute motor, neurocognitive and neurophysiological change following concussion injury in Australian amateur football. A prospective multimodal investigation. J. Sci. Med. Sport 2015, 18, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Dimou, S.; Lagopoulos, J. Toward objective markers of concussion in sport: A review of white matter and neurometabolic changes in the brain after sports-related concussion. J. Neurotraum. 2014, 31, 413–424. [Google Scholar] [CrossRef]
- Daly, E.; Pearce, A.J.; Finnegan, E.; Cooney, C.; McDonagh, M.; Scully, G.; McCann, M.; Doherty, R.; White, A.; Phelan, S.; et al. An assessment of current concussion identification and diagnosis methods in sports settings: A systematic review. BMC Sport. Sci. Med. Rehabil. 2022, 14, 125. [Google Scholar] [CrossRef]
- Fraser, C.L.; Mobbs, R. Visual effects of concussion: A review. Clin. Exp. Ophthalmol. 2022, 50, 104–109. [Google Scholar] [CrossRef]
- Maruta, J.; Suh, M.; Niogi, S.N.; Mukherjee, P.; Ghajar, J. Visual tracking synchronization as a metric for concussion screening. J. Head Trauma Rehabil. 2010, 25, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Orban de Xivry, J.J.; Lefèvre, P. Saccades and pursuit: Two outcomes of a single sensorimotor process. J. Physiol. 2007, 584, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Erkelens, C.J. Coordination of smooth pursuit and saccades. Vis. Res. 2006, 46, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Dursteler, M.; Wurtz, R.; Newsome, W. Directional pursuit deficits following lesions of the foveal representation within the superior temporal sulcus of the macaque monkey. J. Neurophysiol. 1987, 57, 1262–1287. [Google Scholar] [CrossRef] [Green Version]
- Mustari, M.J.; Ono, S. Neural mechanisms for smooth pursuit in strabismus. Ann. N. Y. Acad. Sci. 2011, 1233, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Newsome, W.T.; Wurtz, R.H.; Dursteler, M.; Mikami, A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J. Neurosci. 1985, 5, 825–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behling, S.; Lisberger, S.G. Different mechanisms for modulation of the initiation and steady-state of smooth pursuit eye movements. J. Neurophysiol. 2020, 123, 1265–1276. [Google Scholar] [CrossRef]
- Ono, S. The neuronal basis of on-line visual control in smooth pursuit eye movements. Vis. Res. 2015, 110, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Zahid, A.B.; Hubbard, M.E.; Lockyer, J.; Podolak, O.; Dammavalam, V.M.; Grady, M.; Nance, M.; Scheiman, M.; Samadani, U.; Master, C.L. Eye tracking as a biomarker for concussion in children. Clin. J. Sport Med. 2020, 30, 433–443. [Google Scholar]
- Maruta, J.; Heaton, K.J.; Kryskow, E.M.; Maule, A.L.; Ghajar, J. Dynamic visuomotor synchronization: Quantification of predictive timing. Behav. Res. Methods 2013, 45, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, D.; King, D.; Hume, P.; Clark, T.; Pearce, A.; Gissane, C. Annual baseline King-Devick oculomotor function testing Is needed due to scores varying by age. Sports 2021, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M. Technical Report of the Use of a Novel Eye Tracking System to Measure Impairment Associated with Mild Traumatic Brain Injury. Cureus 2017, 9, e1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Wei, B.; Blakely, S.; Baronia, B.C. Diagnosis and management of mild TBI via eye tracking. Neurology 2022, 98, S14. [Google Scholar] [CrossRef]
- Murray, N.G.; Szekely, B.; Islas, A.; Munkasy, B.; Gore, R.; Berryhill, M.; Reed-Jones, R.J. Smooth pursuit and saccades after sport-related concussion. J. Neurotraum. 2020, 37, 340–346. [Google Scholar] [CrossRef]
- Puckett, Y.; Caballero, B.; Dissanaike, S.; Richmond, R.; Ronaghan, C.A. Surgeons maintain better focus working 12-hour shifts compared to 24-hour calls. J. Surg. Educ. 2021, 78, 1280–1285. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Nguyen, M.; King, D.; Pearce, A. A reliability and comparative analysis of the new randomized King-Devick test. J. Neuroophthalmol. 2020, 40, 207–212. [Google Scholar] [CrossRef]
- Jahnke, N.A.; Still, J.B.; Gamel, G.L.; Baronia, B.C. Method and System for Cognitive Function Testing. U.S. Patent 10,398,301, 3 September 2019. [Google Scholar]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Oberlander, T.J.; Olson, B.L.; Weidauer, L. Test-retest reliability of the King-Devick test in an adolescent population. J. Athl. Train. 2017, 52, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Worts, P.R.; Schatz, P.; Burkhart, S.O. Test performance and test-retest reliability of the vestibular/ocular motor screening and King-Devick test in adolescent athletes during a competitive sport season. Am. J. Sport. Med. 2018, 46, 2004–2010. [Google Scholar] [CrossRef]
- Hecimovich, M.; King, D.; Dempsey, A.R.; Murphy, M. The King-Devick test is a valid and reliable tool for assessing sport-related concussion in Australian football: A prospective cohort study. J. Sci. Med. Sport 2018, 21, 1004–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, M.; Yang, D.; Liu, W. Learning effect and its prediction for cognitive tests used in studies on indoor environmental quality. Energy Build. 2019, 197, 87–98. [Google Scholar] [CrossRef]
- Galetta, K.M.; Liu, M.; Leong, D.F.; Ventura, R.E.; Galetta, S.L.; Balcer, L.J. The King-Devick test of rapid number naming for concussion detection: Meta-analysis and systematic review of the literature. Concussion 2016, 1, CNC8. [Google Scholar] [CrossRef] [Green Version]
- Ionta, S. Visual Neuropsychology in Development: Anatomo-Functional Brain Mechanisms of Action/Perception Binding in Health and Disease. Front. Hum. Neurosci. 2021, 15, 689912. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.O.; Finocchio, D.V.; Ong, L.; Fuchs, A.F. Smooth pursuit in 1-to 4-month-old human infants. Vis. Res. 1997, 37, 3009–3020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R.G.; Radant, A.D.; Hommer, D.W. A Developmental Study of Smooth Pursuit Eye Movements in Normal Children from 7 to 15 Years of Age. J. Am. Acad. Child Adolesc. Psychiatry 1993, 32, 783–791. [Google Scholar] [CrossRef]
- Luna, B.; Velanova, K.; Geier, C.F. Development of eye-movement control. Brain Cogn. 2008, 68, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Doettl, S.M.; McCaslin, D.L. Oculomotor Assessment in Children. Semin. Hear. 2018, 39, 275–287. [Google Scholar]
- Rist, B.; Cohen, A.; Pearce, A.J. King-Devick performance following moderate and high exercise intensity bouts. Int. J. Exerc. Sci. 2017, 10, 619–628. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearce, A.J.; Daly, E.; Ryan, L.; King, D. Reliability of a Smooth Pursuit Eye-Tracking System (EyeGuide Focus) in Healthy Adolescents and Adults. J. Funct. Morphol. Kinesiol. 2023, 8, 83. https://doi.org/10.3390/jfmk8020083
Pearce AJ, Daly E, Ryan L, King D. Reliability of a Smooth Pursuit Eye-Tracking System (EyeGuide Focus) in Healthy Adolescents and Adults. Journal of Functional Morphology and Kinesiology. 2023; 8(2):83. https://doi.org/10.3390/jfmk8020083
Chicago/Turabian StylePearce, Alan J., Ed Daly, Lisa Ryan, and Doug King. 2023. "Reliability of a Smooth Pursuit Eye-Tracking System (EyeGuide Focus) in Healthy Adolescents and Adults" Journal of Functional Morphology and Kinesiology 8, no. 2: 83. https://doi.org/10.3390/jfmk8020083
APA StylePearce, A. J., Daly, E., Ryan, L., & King, D. (2023). Reliability of a Smooth Pursuit Eye-Tracking System (EyeGuide Focus) in Healthy Adolescents and Adults. Journal of Functional Morphology and Kinesiology, 8(2), 83. https://doi.org/10.3390/jfmk8020083







