Omega-3 Index as a Sport Biomarker: Implications for Cardiovascular Health, Injury Prevention, and Athletic Performance
Abstract
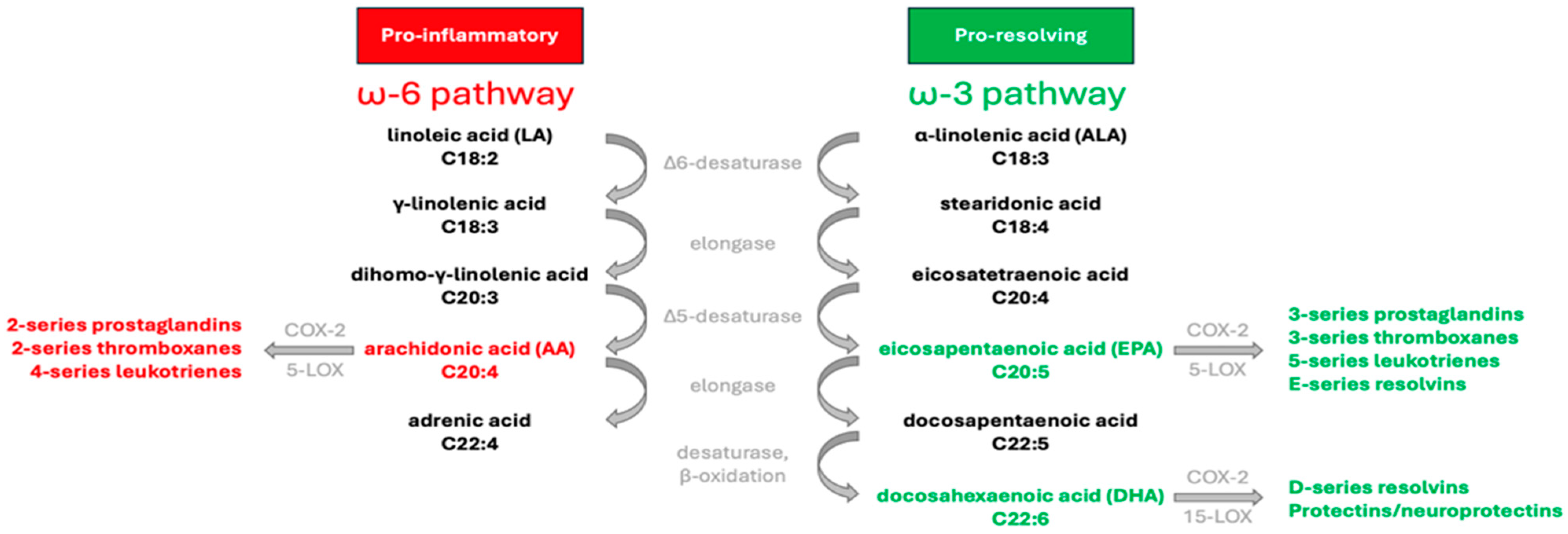
1. Introduction
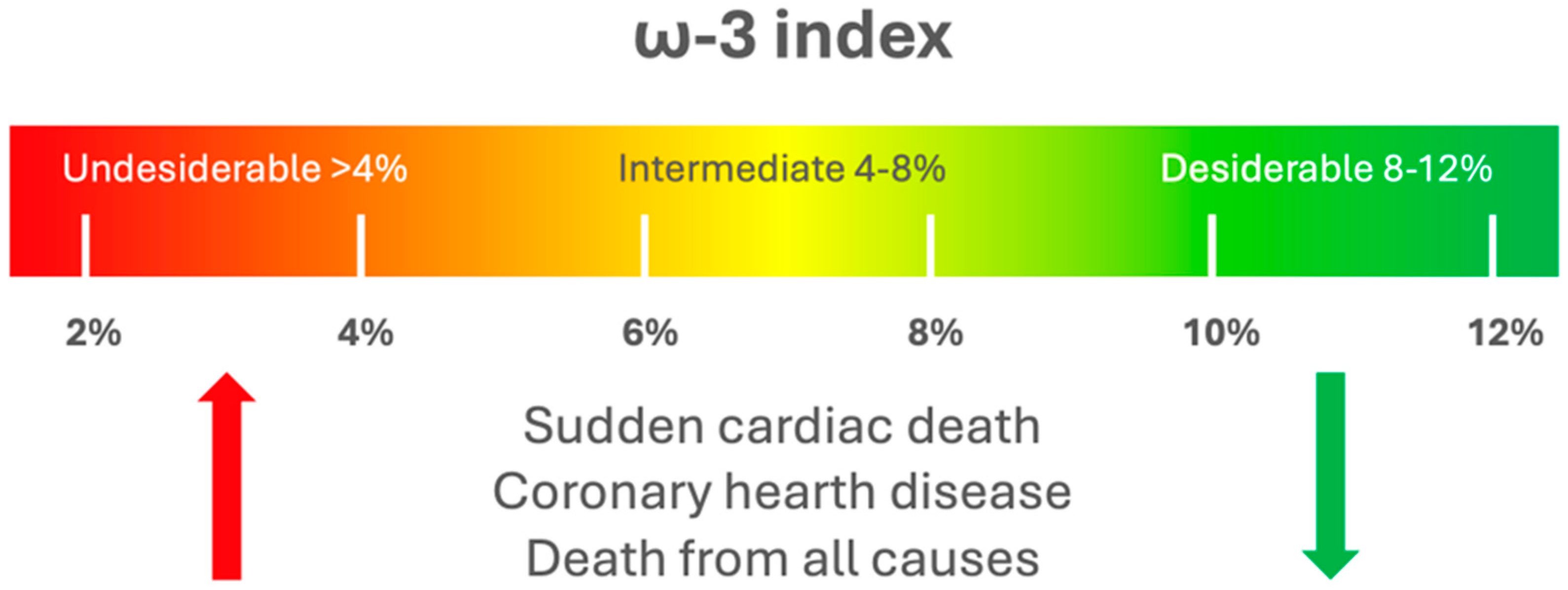
2. ω-3 Index and Cardiovascular Health in Sports
| First Author, Year | Population | Study Design | Intervention with Dosage | Key Findings |
|---|---|---|---|---|
| Anzalone et al., 2019 [34] | 404 NCAA Division I football players | Retrospective, cross-sectional study | N.A. | - Low O3I in 34% of athletes - >8% O3I in no athletes |
| Davinelli et al., 2019 [48] | 257 non-elite runners | Retrospective, observational study | N.A. | - Inverse correlation between O3I and AA/EPA ratio - Gradual decrease in the O3I and increase in AA/EPA ratio with higher weekly running distance |
| Davinelli et al., 2023 [49] | 275 non-elite runners | Retrospective, observational study | N.A. | - Association of high values of O3I with the lowest number of running-related injuries |
| Drobnic et al., 2017 [41] | 24 summer sports athletes | Randomized, parallel-group study | Supplementation with 760 mg/day or 1140 mg/day ω-3 PUFA for 4 months | - Dose-dependent increase in the content of EPA and DHA in the red blood cells at 4 months - Greater increment in O3I in athletes with lower basal levels |
| Heileson et al., 2021 [50] | 66 NCAA American football athletes | Multi-site, non-randomized, parallel-group study | Supplementation with 2000 mg DHA, 560 mg EPA, or 320 mg DPA 4 times per week for a total of 89 days | - Increase in O3I and reduced elevation in serum NF-L levels after supplementation |
| Hingley et al., 2017 [51] | 26 trained male subjects | Double-blind, placebo-controlled study | Supplementation with 560 mg DHA and 140 mg EPA/day for 8 weeks | - Increase in O3I and reduced relative oxygen consumption during the cycling time trial after supplementation |
| Jaworska et al., 2023 [47] | 24 male long-distance runners | Randomized, placebo-controlled study | Supplementation with 3 g of ω-3 PUFA for 3 weeks | - Improvement in blood lipid profiles and O3I - Reduction in inflammation mediators and cardiac damage markers after the eccentric exercise tests |
| Larkin et al., 2024 [43] | 47 American college football players | Longitudinal and cross-sectional study | Supplementation with algae oil each weekend for 5 weeks (equivalent to 750 mg of DHA and 375 mg EPA per day) | - Improvement in both low baseline O3I and high AA/EPA ratio with body mass-specific dose effects |
| Lembke et al., 2014 [52] | 69 male and female college students | Randomized, placebo-controlled study | Supplementation with 2.7 g ω-3 PUFA/day for 30 days | - Less pain related to DOMS following heavy exercise at 72 and 96 h in subjects with a higher O3I reported - Lower serum levels of blood lactate in subjects with a high O3I - Reduction in CRP at 24 h in high O3I subjects |
| Lust et al., 2023 [44] | 69 American football players | Randomized, double-blind, placebo-controlled, parallel-group study | Supplementation with 2, 4, or 6 g/day of DHA for 27 weeks | - Dose–response incorporation of DHA into RBC membranes up to 6 g/day - Achievement of >8% O3I in athletes in 8 weeks with 6 g/day of DHA supplementation |
| MacArtney et al., 2014 [53] | 39 physically fit and healthy males | Double-blind, parallel-group study | Supplementation with 140 mg of EPA and 560 mg of DHA/day for 8 weeks | - Increase in O3I, reduction in mean heart rate during exercise, and improved heart rate recovery after supplementation |
| Tomczyk et al., 2023 [54] | 26 amateur male long-distance runners | Randomized, parallel-group study | Supplementation with 2234 mg of EPA and 916 mg DHA/day for 12 weeks | - Increase in O3I and indicators of running performance, including running economy and peak oxygen uptake |
| Zebrowska et al., 2021 [46] | 24 recreational marathon runners | Randomized, blind, placebo-controlled study | Supplementation with 852 mg EPA, 1602 mg DHA, and 12 mg and 30 µg of vitamin E and D/day for 3 weeks | - Increase in O3I and decrease in AA/EPA ratio after supplementation - Positive changes in lipid composition of erythrocytes, serum adipocytokines, and post-exercise proinflammatory cytokine levels |
3. ω-3 Index and Sports Injury Prevention
4. ω-3 Index and Sport Performance
4.1. ω-3 Index and Strength and Power
4.2. ω-3 Index and Endurance Performance
4.3. ω-3 Index and Exercise-Induced Fatigue
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catalá, A. Five Decades with Polyunsaturated Fatty Acids: Chemical Synthesis, Enzymatic Formation, Lipid Peroxidation and Its Biological Effects. J. Lipids 2013, 2013, 710290. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Mougios, V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med. 2004, 34, 1051–1076. [Google Scholar] [CrossRef]
- Martorell, M.; Capó, X.; Bibiloni, M.M.; Sureda, A.; Mestre-Alfaro, A.; Batle, J.M.; Llompart, I.; Tur, J.A.; Pons, A. Docosahexaenoic acid supplementation promotes erythrocyte antioxidant defense and reduces protein nitrosative damage in male athletes. Lipids 2015, 50, 131–148. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Kohrt, W.M. Regulation of carbohydrate and fat metabolism during and after exercise. Annu. Rev. Nutr. 1996, 16, 121–138. [Google Scholar] [CrossRef]
- Tepsic, J.; Vucic, V.; Arsic, A.; Blazencic-Mladenovic, V.; Mazic, S.; Glibetic, M. Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur. J. Appl. Physiol. 2009, 107, 359–365. [Google Scholar] [CrossRef]
- Ney, J.G.; Koury, J.C.; Azeredo, V.B.; Casimiro-Lopes, G.; Trugo, N.M.F.; Torres, A.G. Associations of n-6 and n-3 polyunsaturated fatty acids and tocopherols with proxies of membrane stability and subcutaneous fat sites in male elite swimmers. Nutr. Res. 2009, 29, 623–630. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins. Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Davinelli, S.; Intrieri, M.; Corbi, G.; Scapagnini, G. Metabolic indices of polyunsaturated fatty acids: Current evidence, research controversies, and clinical utility. Crit. Rev. Food Sci. Nutr. 2021, 61, 259–274. [Google Scholar] [CrossRef]
- Von Schacky, C.; Fischer, S.; Weber, P.C. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J. Clin. Investig. 1985, 76, 1626–1631. [Google Scholar] [CrossRef]
- Harris, W.S.; Thomas, R.M. Biological variability of blood omega-3 biomarkers. Clin. Biochem. 2010, 43, 338–340. [Google Scholar] [CrossRef]
- Harris, W.S.; Pottala, J.V.; Varvel, S.A.; Borowski, J.J.; Ward, J.N.; McConnell, J.P. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: Observations from 160,000 patients. Prostaglandins. Leukot. Essent. Fatty Acids 2013, 88, 257–263. [Google Scholar] [CrossRef]
- Ali, S.; Intrieri, M.; Pisanti, A.; Cardinale, G.; Corbi, G.; Scapagnini, G.; Davinelli, S. Determination of n-3 index and arachidonic acid/eicosapentaenoic acid ratio in dried blood spot by gas chromatography. Biotechniques 2022, 73, 25–33. [Google Scholar] [CrossRef]
- Marangoni, F.; Colombo, C.; Galli, C. A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: Applicability to nutritional and epidemiological studies. Anal. Biochem. 2004, 326, 267–272. [Google Scholar] [CrossRef]
- Baylin, A.; Campos, H. The use of fatty acid biomarkers to reflect dietary intake. Curr. Opin. Lipidol. 2006, 17, 22–27. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Montorfano, G.; Negroni, M.; Adorni, L.; Berselli, P.; Corsetto, P.; Wahle, K.; Berra, B. A rapid method for determining arachidonic:eicosapentaenoic acid ratios in whole blood lipids: Correlation with erythrocyte membrane ratios and validation in a large Italian population of various ages and pathologies. Lipids Health Dis. 2010, 9, 7. [Google Scholar] [CrossRef]
- Fenton, J.I.; Gurzell, E.A.; Davidson, E.A.; Harris, W.S. Red blood cell PUFAs reflect the phospholipid PUFA composition of major organs. Prostaglandins. Leukot. Essent. Fatty Acids 2016, 112, 12–23. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chen, J.; Chen, Y.; Harris, W.S. Myocardial infarction does not affect fatty-acid profiles in rats. Prostaglandins. Leukot. Essent. Fatty Acids 2009, 81, 411–416. [Google Scholar] [CrossRef]
- Von Schacky, C. Omega-3 fatty acids in cardiovascular disease—An uphill battle. Prostaglandins. Leukot. Essent. Fatty Acids 2015, 92, 41–47. [Google Scholar] [CrossRef]
- Von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef]
- Mickleborough, T.D. Omega-3 polyunsaturated fatty acids in physical performance optimization. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 83–96. [Google Scholar] [CrossRef]
- Bryhn, M. Prevention of Sports Injuries by Marine Omega-3 Fatty Acids. J. Am. Coll. Nutr. 2015, 34 (Suppl. 1), 60–61. [Google Scholar] [CrossRef]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta 2012, 1818, 1309–1317. [Google Scholar] [CrossRef]
- Santos, V.C.; Levada-Pires, A.C.; Alves, S.R.; Pithon-Curi, T.C.; Curi, R.; Cury-Boaventura, M.F. Effects of DHA-Rich Fish Oil Supplementation on Lymphocyte Function Before and After a Marathon Race. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 161–169. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [CrossRef]
- Shei, R.-J.; Lindley, M.R.; Mickleborough, T.D. Omega-3 Polyunsaturated Fatty Acids in the Optimization of Physical Performance. Mil. Med. 2014, 179, 144–156. [Google Scholar] [CrossRef]
- Walser, B.; Giordano, R.M.; Stebbins, C.L. Supplementation with omega-3 polyunsaturated fatty acids augments brachial artery dilation and blood flow during forearm contraction. Eur. J. Appl. Physiol. 2006, 97, 347–354. [Google Scholar] [CrossRef]
- Tribulova, N.; Bacova, B.S.; Benova, T.E.; Knezl, V.; Barancik, M.; Slezak, J. Omega-3 Index and Anti-Arrhythmic Potential of Omega-3 PUFAs. Nutrients 2017, 9, 1191. [Google Scholar] [CrossRef]
- Metcalf, R.G.; Cleland, L.G.; Gibson, R.A.; Roberts-Thomson, K.C.; Edwards, J.R.M.; Sanders, P.; Stuklis, R.; James, M.J.; Young, G.D. Relation between blood and atrial fatty acids in patients undergoing cardiac bypass surgery. Am. J. Clin. Nutr. 2010, 91, 528–534. [Google Scholar] [CrossRef]
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef]
- Baranauskas, M.; Stukas, R.; Tubelis, L.; Žagminas, K.; Šurkiene, G.; Švedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravičius, J.A. Nutritional habits among high-performance endurance athletes. Medicina 2015, 51, 351–362. [Google Scholar] [CrossRef]
- Von Schacky, C.; Kemper, M.; Haslbauer, R.; Halle, M. Low Omega-3 Index in 106 German elite winter endurance athletes: A pilot study. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 559–564. [Google Scholar] [CrossRef]
- Anzalone, A.; Carbuhn, A.; Jones, L.; Gallop, A.; Smith, A.; Johnson, P.; Swearingen, L.; Moore, C.; Rimer, E.; McBeth, J.; et al. The Omega-3 Index in National Collegiate Athletic Association Division I Collegiate Football Athletes. J. Athl. Train. 2019, 54, 7–11. [Google Scholar] [CrossRef]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef]
- Aarsetøy, H.; Pönitz, V.; Nilsen, O.B.; Grundt, H.; Harris, W.S.; Nilsen, D.W.T. Low levels of cellular omega-3 increase the risk of ventricular fibrillation during the acute ischaemic phase of a myocardial infarction. Resuscitation 2008, 78, 258–264. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; Van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Longstreth, W.T.; Lemaitre, R.N.; Manolio, T.A.; Kuller, L.H.; Burke, G.L.; Siscovick, D.S. Fish Consumption and Stroke Risk in Elderly Individuals: The Cardiovascular Health Study. Arch. Intern. Med. 2005, 165, 200. [Google Scholar] [CrossRef]
- Marchioli, R. Dietary supplementation with N-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Thielecke, F.; Blannin, A. Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients 2020, 12, 3712. [Google Scholar] [CrossRef]
- Drobnic, F.; Rueda, F.; Pons, V.; Banquells, M.; Cordobilla, B.; Domingo, J.C. Erythrocyte Omega-3 Fatty Acid Content in Elite Athletes in Response to Omega-3 Supplementation: A Dose-Response Pilot Study. J. Lipids 2017, 2017, 1472719. [Google Scholar] [CrossRef]
- Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Etherton, T.D.; Fleming, J.A.; Kris-Etherton, P.M. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: A dose-response randomized controlled trial. J. Am. Heart Assoc. 2013, 2, e000513. [Google Scholar] [CrossRef]
- Larkin, T.A.; McKay, B.; Sampson, J.A.; Delaney, J.; Murray, A.; Pedlar, C.R.; Lewis, N.A.; Peoples, G.E. A Low Omega-3 Index and High AA/EPA Ratio in American College Football Players are Both Improved Following 5 Weeks of DHA-Rich Algae Oil Supplementation. J. Sci. Sport Exerc. 2024, 1–9. [Google Scholar] [CrossRef]
- Lust, C.A.C.; Burns, J.L.; Jones, M.T.; Smith, S.B.; Choi, S.H.O.; Krk, M.; Gable, D.A.; Oliver, J.M.; Ma, D.W.L. The Dose-Response Effect of Docosahexaenoic Acid on the Omega-3 Index in American Football Athletes. Med. Sci. Sports Exerc. 2023, 55, 865–872. [Google Scholar] [CrossRef]
- Buckley, J.D.; Burgess, S.; Murphy, K.J.; Howe, P.R.C. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J. Sci. Med. Sport 2009, 12, 503–507. [Google Scholar] [CrossRef]
- Żebrowska, A.; Hall, B.; Stolecka-Warzecha, A.; Stanula, A.; Sadowska-Krępa, E. The Effect of Omega-3 Fatty Acid Supplementation on Serum Adipocytokines, Lipid Profile and Biochemical Markers of Inflammation in Recreational Runners. Nutrients 2021, 13, 456. [Google Scholar] [CrossRef]
- Jaworska, M.; Siatkowski, S.; Żebrowska, A. The Effects of Omega-3 Fatty Acid Supplementation on the Lipid Profile and Cardiovascular Markers Following Downhill Running in Long-Distance Runners. J. Hum. Kinet. 2023, 89, 123. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Corbi, G.; Righetti, S.; Casiraghi, E.; Chiappero, F.; Martegani, S.; Pina, R.; De Vivo, I.; Simopoulos, A.P.; Scapagnini, G. Relationship between distance run per week, omega-3 index, and arachidonic acid (AA)/eicosapentaenoic acid (EPA) ratio: An Observational Retrospective Study in Non-elite Runners. Front. Physiol. 2019, 10, 435605. [Google Scholar] [CrossRef]
- Davinelli, S.; Intrieri, M.; Ali, S.; Righetti, S.; Mondazzi, L.; Scapagnini, G.; Corbi, G. Omega-3 index and AA/EPA ratio as biomarkers of running-related injuries: An observational study in recreational runners. Eur. J. Sport Sci. 2023, 23, 134–142. [Google Scholar] [CrossRef]
- Heileson, J.L.; Anzalone, A.J.; Carbuhn, A.F.; Askow, A.T.; Stone, J.D.; Turner, S.M.; Hillyer, L.M.; Ma, D.W.L.; Luedke, J.A.; Jagim, A.R.; et al. The effect of omega-3 fatty acids on a biomarker of head trauma in NCAA football athletes: A multi-site, non-randomized study. J. Int. Soc. Sports Nutr. 2021, 18, 65. [Google Scholar] [CrossRef]
- Hingley, L.; Macartney, M.J.; Brown, M.A.; McLennan, P.L.; Peoples, G.E. DHA-rich Fish Oil Increases the Omega-3 Index and Lowers the Oxygen Cost of Physiologically Stressful Cycling in Trained Individuals. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 335–343. [Google Scholar] [CrossRef]
- Lembke, P.; Capodice, J.; Hebert, K.; Swenson, T. Influence of Omega-3 (N3) Index on Performance and Wellbeing in Young Adults after Heavy Eccentric Exercise. J. Sports Sci. Med. 2014, 13, 151. [Google Scholar]
- MacArtney, M.J.; Hingley, L.; Brown, M.A.; Peoples, G.E.; McLennan, P.L. Intrinsic heart rate recovery after dynamic exercise is improved with an increased omega-3 index in healthy males. Br. J. Nutr. 2014, 112, 1984–1992. [Google Scholar] [CrossRef]
- Tomczyk, M.; Jost, Z.; Chroboczek, M.; Urbański, R.; Calder, P.C.; Fisk, H.L.; Sprengel, M.; Antosiewicz, J. Effects of 12 Wk of Omega-3 Fatty Acid Supplementation in Long-Distance Runners. Med. Sci. Sports Exerc. 2023, 55, 216–224. [Google Scholar] [CrossRef]
- Ryan, J.L.; Pracht, E.E.; Orban, B.L. Inpatient and emergency department costs from sports injuries among youth aged 5–18 years. BMJ Open Sport—Exerc. Med. 2019, 5, 491. [Google Scholar] [CrossRef]
- Wall, B.T.; van Loon, L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef]
- Seki, K.; Taniguchi, Y.; Narusawa, M. Effects of joint immobilization on firing rate modulation of human motor units. J. Physiol. 2001, 530, 507. [Google Scholar] [CrossRef]
- Close, G.L.; Baar, K.; Sale, C.; Bermon, S. Nutrition for the Prevention and Treatment of Injuries in Track and Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 189–197. [Google Scholar] [CrossRef]
- Fiorilli, G.; Buonsenso, A.; Centorbi, M.; Calcagno, G.; Iuliano, E.; Angiolillo, A.; Ciccotelli, S.; di Cagno, A.; Di Costanzo, A. Long term physical activity improves quality of life perception, healthy nutrition, and daily life management in elderly: A randomized controlled trial. Nutrients 2022, 14, 2527. [Google Scholar] [CrossRef]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sports Med. 2015, 45 (Suppl. 1), 93–104. [Google Scholar] [CrossRef]
- Smith, C.; Kruger, M.J.; Smith, R.M.; Myburgh, K.H. The inflammatory response to skeletal muscle injury: Illuminating complexities. Sports Med. 2008, 38, 947–969. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 fatty acids supplementation attenuates inflammatory markers after eccentric exercise in untrained men. Clin. J. Sport Med. 2011, 21, 131–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Joshi-Barve, S.; Barve, S.; Chen, L.H. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J. Am. Coll. Nutr. 2004, 23, 71–78. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Vassiliou, E.; Adhikary, S.; Toscano, M.G.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010, 9, 12. [Google Scholar] [CrossRef]
- Daneshvar, D.H.; Nowinski, C.J.; Mckee, A.C.; Cantu, R.C. The Epidemiology of Sport-Related Concussion. Clin. Sports Med. 2011, 30, 1. [Google Scholar] [CrossRef]
- Oliver, J.M.; Jones, M.T.; Kirk, K.M.; Gable, D.A.; Repshas, J.T.; Johnson, T.A.; Andréasson, U.; Norgren, N.; Blennow, K.; Zetterberg, H. Serum Neurofilament Light in American Football Athletes over the Course of a Season. J. Neurotrauma 2016, 33, 1784–1789. [Google Scholar] [CrossRef]
- Oliver, J.M.; Anzalone, A.J.; Turner, S.M. Protection Before Impact: The Potential Neuroprotective Role of Nutritional Supplementation in Sports-Related Head Trauma. Sports Med. 2018, 48, 39–52. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Exercise facilitates the action of dietary DHA on functional recovery after brain trauma. Neuroscience 2013, 248, 655–663. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary strategy to repair plasma membrane after brain trauma: Implications for plasticity and cognition. Neurorehabil. Neural Repair 2014, 28, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Kevala, K.; Kim, H.Y. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS ONE 2014, 9, e86472. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.L.; Berman, N.E.J.; Levant, B. Low brain DHA content worsens sensorimotor outcomes after TBI and decreases TBI-induced Timp1 expression in juvenile rats. Prostaglandins. Leukot. Essent. Fatty Acids 2013, 89, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Oliver, J.M.; Jones, M.T.; Kirk, K.M.; Gable, D.A.; Repshas, J.T.; Johnson, T.A.; Andréasson, U.; Norgren, N.; Blennow, K.; Zetterberg, H. Effect of Docosahexaenoic Acid on a Biomarker of Head Trauma in American Football. Med. Sci. Sports Exerc. 2016, 48, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Cabrera, D.; Knafo, S.; Thomas, J.; Peacock, C.; Tartar, J. Neurofilament Light (NFL) in Division II Female Soccer Players: A Potential Biomarker for Brain Trauma. J. Exerc. Physiol. Online 2021, 24, 1. [Google Scholar]
- Hudek, R.; von Schacky, C.; Passow, A.; Abdelkawi, A.F.; Werner, B.; Gohlke, F. Degenerative rotator cuff tears are associated with a low Omega-3 Index. Prostaglandins. Leukot. Essent. Fatty Acids 2019, 148, 35–40. [Google Scholar] [CrossRef]
- Voloshin, I.; Gelinas, J.; Maloney, M.D.; O’Keefe, R.J.; Bigliani, L.U.; Blaine, T.A. Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthroscopy 2005, 21, 1076.e1–1076.e9. [Google Scholar] [CrossRef]
- Moir, H.J.; Maciejczyk, M.; Maciejczyk, M.; Aidar, F.J.; Arazi, H. Editorial: Exercise-induced oxidative stress and the role of antioxidants in sport and exercise. Front. Sport. Act. Living 2023, 5, 1269826. [Google Scholar] [CrossRef]
- Buonocore, D.; Verri, M.; Giolitto, A.; Doria, E.; Ghitti, M.; Dossena, M. Effect of 8-week n-3 fatty-acid supplementation on oxidative stress and inflammation in middle- and long-distance running athletes: A pilot study. J. Int. Soc. Sports Nutr. 2020, 17, 55. [Google Scholar] [CrossRef]
- Gerlach, K.E.; Burton, H.W.; Dorn, J.M.; Leddy, J.J.; Horvath, P.J. Fat intake and injury in female runners. J. Int. Soc. Sports Nutr. 2008, 5, 1. [Google Scholar] [CrossRef]
- Kamolrat, T.; Gray, S.R. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem. Biophys. Res. Commun. 2013, 432, 593–598. [Google Scholar] [CrossRef]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef]
- McBurney, M.I.; Tintle, N.L.; Harris, W.S. Omega-3 index is directly associated with a healthy red blood cell distribution width. Prostaglandins Leukot. Essent. Fat. Acids 2022, 176, 102376. [Google Scholar] [CrossRef]
- Mcglory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.R.; Hamilton, D.L.; et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 2016, 4, e12715. [Google Scholar] [CrossRef]
- Bhullar, A.S.; Putman, C.T.; Mazurak, V.C. Potential Role of Omega-3 Fatty Acids on the Myogenic Program of Satellite Cells. Nutr. Metab. Insights 2016, 9, 1–10. [Google Scholar] [CrossRef]
- McGlory, C.; Galloway, S.D.R.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 199–206. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef]
- Lv, Z.T.; Zhang, J.M.; Zhu, W.T. Omega-3 Polyunsaturated Fatty Acid Supplementation for Reducing Muscle Soreness after Eccentric Exercise: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2020, 2020, 8062017. [Google Scholar] [CrossRef]
- Gravina, L.; Brown, F.F.; Alexander, L.; Dick, J.; Bell, G.; Witard, O.C.; Galloway, S.D.R. n-3 Fatty Acid Supplementation During 4 Weeks of Training Leads to Improved Anaerobic Endurance Capacity, but not Maximal Strength, Speed, or Power in Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 305–313. [Google Scholar] [CrossRef][Green Version]
- Gammone, M.A.; Gemello, E.; Riccioni, G.; D’Orazio, N. Marine bioactives and potential application in sports. Mar. Drugs 2014, 12, 2357–2382. [Google Scholar] [CrossRef]
- Lanza, I.R.; Blachnio-Zabielska, A.; Johnson, M.L.; Schimke, J.M.; Jakaitis, D.R.; Lebrasseur, N.K.; Jensen, M.D.; Sreekumaran Nair, K.; Zabielski, P. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1391–E1403. [Google Scholar] [CrossRef]
- Watt, M.J.; Heigenhauser, G.J.F.; Dyck, D.J.; Spriet, L.L. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J. Physiol. 2002, 541, 969. [Google Scholar] [CrossRef]
- Kawabata, F.; Neya, M.; Hamazaki, K.; Watanabe, Y.; Kobayashi, S.; Tsuji, T. Supplementation with eicosapentaenoic acid-rich fish oil improves exercise economy and reduces perceived exertion during submaximal steady-state exercise in normal healthy untrained men. Biosci. Biotechnol. Biochem. 2014, 78, 2081–2088. [Google Scholar] [CrossRef]
- Peoples, G.E.; McLennan, P.L.; Howe, P.R.C.; Groeller, H. Fish oil reduces heart rate and oxygen consumption during exercise. J. Cardiovasc. Pharmacol. 2008, 52, 540–547. [Google Scholar] [CrossRef]
- Żebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gąsior, Z.; Poprzęcki, S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur. J. Sport Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Role of lipoxins, resolvins, and other bioactive lipids in colon and pancreatic cancer. Cancer Metastasis Rev. 2011, 30, 507–523. [Google Scholar] [CrossRef]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef]
- Panza, V.S.P.; Diefenthaeler, F.; da Silva, E.L. Benefits of dietary phytochemical supplementation on eccentric exercise-induced muscle damage: Is including antioxidants enough? Nutrition 2015, 31, 1072–1082. [Google Scholar] [CrossRef]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. Eur. J. Appl. Physiol. 2017, 117, 575–582. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. The Effects of Ingestion of Omega-3 Fatty Acids on Perceived Pain and External Symptoms of Delayed Onset Muscle Soreness in Untrained Men. Clin. J. Sport Med. 2009, 19, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Jouris, K.B.; Mcdaniel, J.L.; Weiss, E.P. The effect of omega-3 fatty acid supplementation on the inflammatory response to eccentric strength exercise. J. Sport. Sci. Med. 2011, 10, 432–438. [Google Scholar]
- Black, K.E.; Witard, O.C.; Baker, D.; Healey, P.; Lewis, V.; Tavares, F.; Christensen, S.; Pease, T.; Smith, B. Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. Eur. J. Sport Sci. 2018, 18, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- McKinley-Barnard, S.K.; Andre, T.L.; Gann, J.J.; Hwang, P.S.; Willoughby, D.S. Effectiveness of fish oil supplementation in attenuating exercise-induced muscle damage in women during midfollicular and midluteal menstrual phases. J. Strength Cond. Res. 2018, 32, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Lenn, J.; Uhl, T.; Mattacola, C.; Boissonneault, G.; Yates, J.; Ibrahim, W.; Bruckner, G. The effects of fish oil and isoflavones on delayed onset muscle soreness. Med. Sci. Sports Exerc. 2002, 34, 1605–1613. [Google Scholar] [CrossRef]
- Gray, P.; Chappell, A.; Jenkinson, A.M.E.; Thies, F.; Gray, S.R. Fish Oil Supplementation Reduces Markers of Oxidative Stress But Not Muscle Soreness After Eccentric Exercise. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 206–214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medoro, A.; Buonsenso, A.; Centorbi, M.; Calcagno, G.; Scapagnini, G.; Fiorilli, G.; Davinelli, S. Omega-3 Index as a Sport Biomarker: Implications for Cardiovascular Health, Injury Prevention, and Athletic Performance. J. Funct. Morphol. Kinesiol. 2024, 9, 91. https://doi.org/10.3390/jfmk9020091
Medoro A, Buonsenso A, Centorbi M, Calcagno G, Scapagnini G, Fiorilli G, Davinelli S. Omega-3 Index as a Sport Biomarker: Implications for Cardiovascular Health, Injury Prevention, and Athletic Performance. Journal of Functional Morphology and Kinesiology. 2024; 9(2):91. https://doi.org/10.3390/jfmk9020091
Chicago/Turabian StyleMedoro, Alessandro, Andrea Buonsenso, Marco Centorbi, Giuseppe Calcagno, Giovanni Scapagnini, Giovanni Fiorilli, and Sergio Davinelli. 2024. "Omega-3 Index as a Sport Biomarker: Implications for Cardiovascular Health, Injury Prevention, and Athletic Performance" Journal of Functional Morphology and Kinesiology 9, no. 2: 91. https://doi.org/10.3390/jfmk9020091
APA StyleMedoro, A., Buonsenso, A., Centorbi, M., Calcagno, G., Scapagnini, G., Fiorilli, G., & Davinelli, S. (2024). Omega-3 Index as a Sport Biomarker: Implications for Cardiovascular Health, Injury Prevention, and Athletic Performance. Journal of Functional Morphology and Kinesiology, 9(2), 91. https://doi.org/10.3390/jfmk9020091











