Erosion of Stumble Correction Evoked with Superficial Peroneal Nerve Stimulation in Older Adults during Walking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Nerve Stimulation
2.4. Electromyography
2.5. Kinematics and Gait Parameters
2.6. Data Acquisition and Analysis
2.7. Statistics
3. Results
3.1. Reflexes
3.1.1. Toe Clearance Reflexes
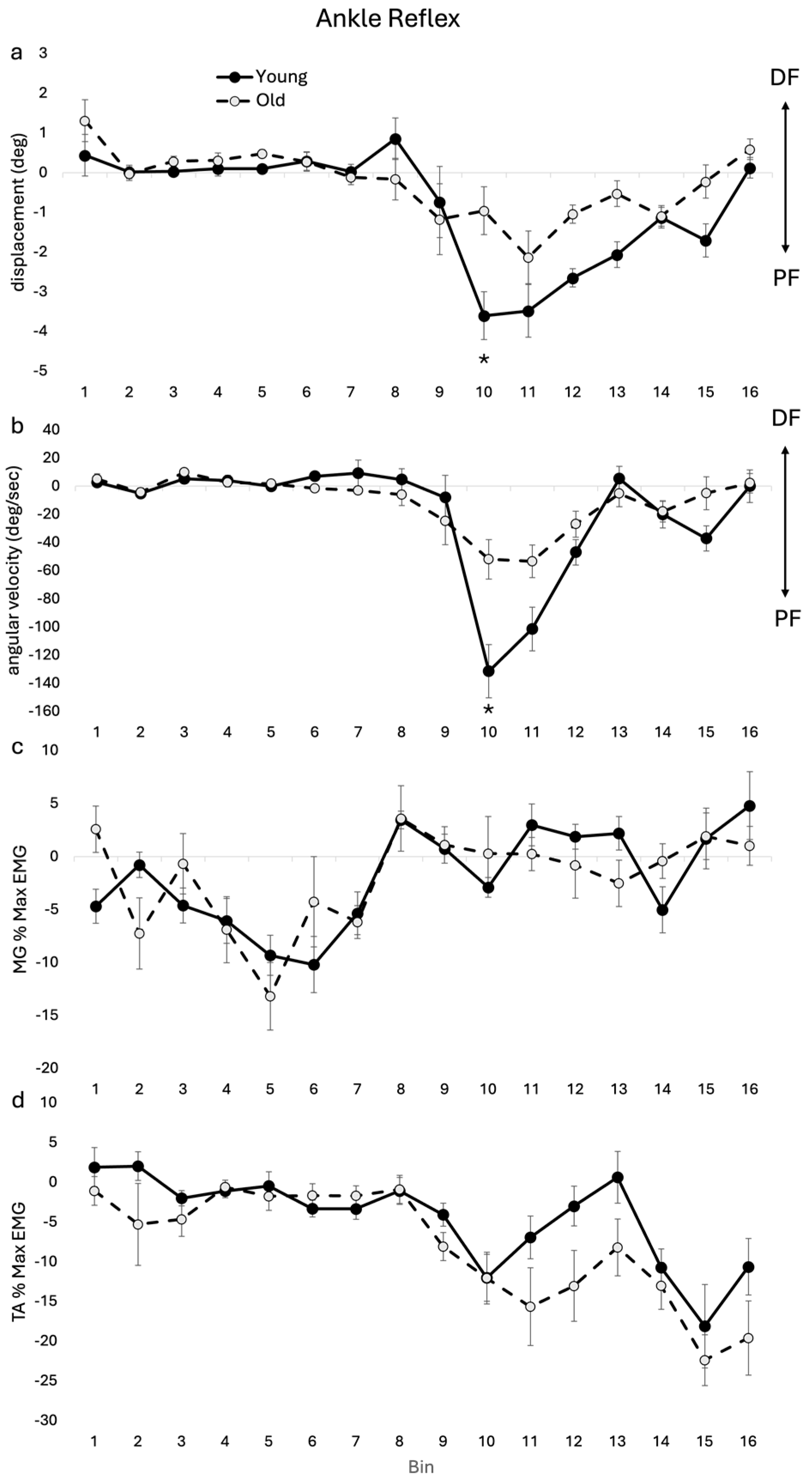
3.1.2. Ankle Kinematic and EMG Reflexes
3.1.3. Knee Kinematic and EMG Reflexes
3.2. Background
3.2.1. Background Toe Clearance
3.2.2. Background Ankle Kinematics and EMG
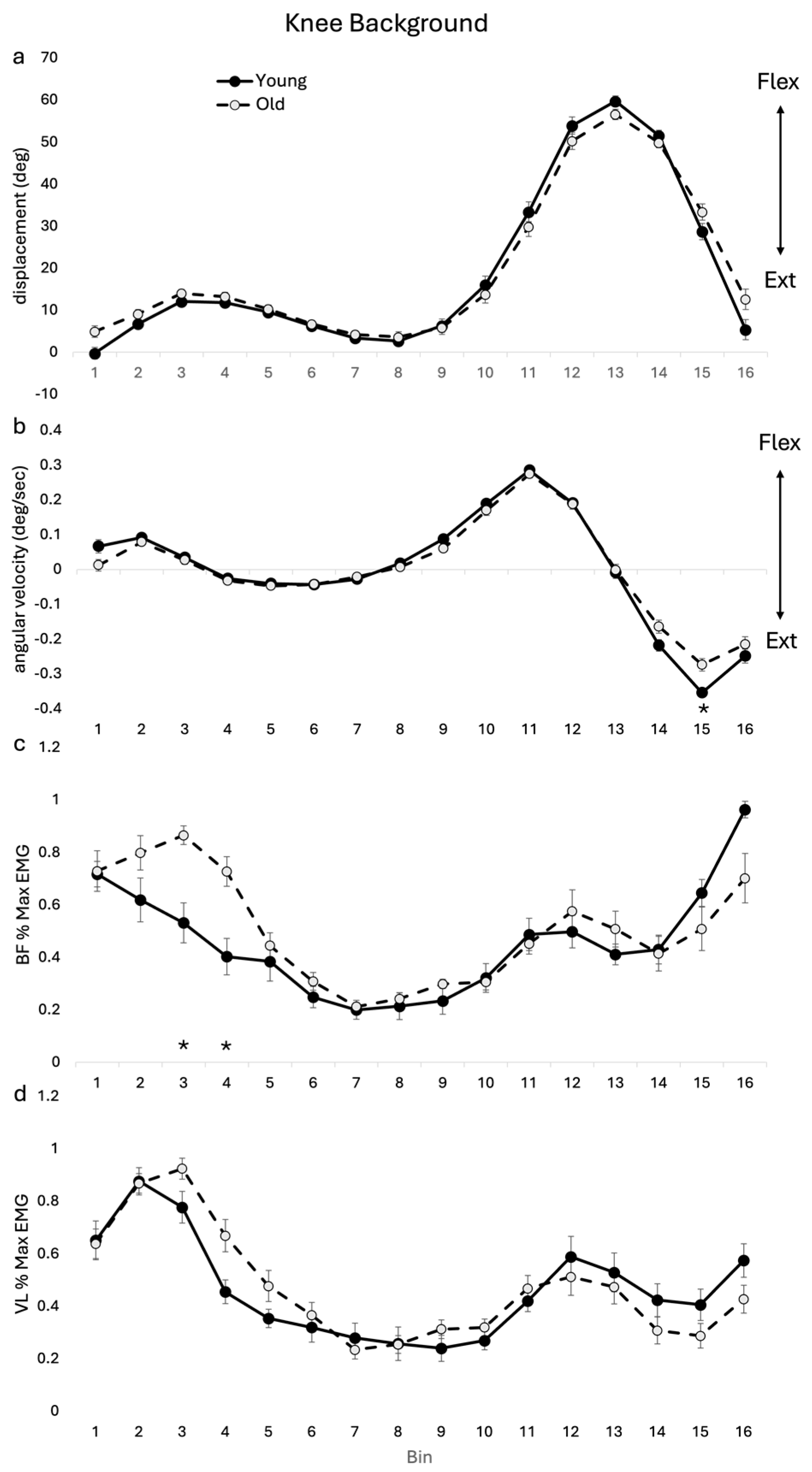
3.2.3. Background Knee Kinematics and EMG
3.3. Combined Reflexes and Background
4. Discussion
4.1. Toe Clearance
4.2. Ankle
4.3. Knee
4.4. Clinical Implications
4.5. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granata, K.P.; Lockhart, T.E. Dynamic Stability Differences in Fall-Prone and Healthy Adults. J. Electromyogr. Kinesiol. 2008, 18, 172–178. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Effects of Walking Speed, Strength and Range of Motion on Gait Stability in Healthy Older Adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, J.L.; Robitaille, Y.; Boivin, J.F.; Suissa, S. Incidence of and Risk Factors for Falls and Injurious Falls among the Community-Dwelling Elderly. Am. J. Epidemiol. 1993, 137, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Fujita, K.; Stein, R.B. Reflexes from the Superficial Peroneal Nerve during Walking in Stroke Subjects. J. Neurophysiol. 1998, 79, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Hundza, S.R.; Zehr, E.P. Muscle Activation and Cutaneous Reflex Modulation during Rhythmic and Discrete Arm Tasks in Orthopaedic Shoulder Instability. Exp. Brain Res. 2007, 179, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Loadman, P.M.; Hundza, S.R. Neural Control of Rhythmic Arm Cycling after Stroke. J. Neurophysiol. 2012, 108, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Duysens, J.; Tax, A.A.M.; Trippel, M.; Dietz, V. Phase-Dependent Reversal of Reflexly Induced Movements during Human Gait. Exp. Brain Res. 1992, 90, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Komiyama, T.; Stein, R.B. Cutaneous Reflexes during Human Gait: Electromyographic and Kinematic Responses to Electrical Stimulation. J. Neurophysiol. 1997, 77, 3311–3325. [Google Scholar] [CrossRef]
- Zehr, E.P.; Stein, R.B. What Functions Do Reflexes Serve during Human Locomotion? Prog. Neurobiol. 1999, 58, 185–205. [Google Scholar] [CrossRef]
- Eng, J.J.; Winter, D.A.; Patla, A.E. Strategies for Recovery from a Trip in Early and Late Swing during Human Walking. Exp. Brain Res. 1994, 102, 339–349. [Google Scholar] [CrossRef]
- Schillings, A.M.; Van Wezel, B.M.H.; Duysens, J. Mechanically Induced Stumbling during Human Treadmill Walking. J. Neurosci. Methods 1996, 67, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Schillings, A.M.; Mulder, T.H.; Duysens, J. Stumbling over Obstacles in Older Adults Compared to Young Adults. J. Neurophysiol. 2005, 94, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.M.; Barrett, R.S.; Morrison, S. Toe Clearance Variability during Walking in Young and Elderly Men. Gait Posture 2008, 28, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hundza, S.R.; Gaur, A.; Brodie, R.; Commandeur, D.; Klimstra, M.D. Age-Related Erosion of Obstacle Avoidance Reflexes Evoked with Electrical Stimulation of Tibial Nerve during Walking. J. Neurophysiol. 2018, 119, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.D.; Ghoussayni, S.N.; Ewins, D.J.; Kent, J.A. A Six Degrees-of-Freedom Marker Set for Gait Analysis: Repeatability and Comparison with a Modified Helen Hayes Set. Gait Posture 2009, 30, 173–180. [Google Scholar] [CrossRef]
- Cavanagh, P.R.; Komi, P.V. Electromechanical Delay in Human Skeletal Muscle under Concentric and Eccentric Contractions. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 42, 159–163. [Google Scholar] [CrossRef]
- Barrett, R.S.; Mills, P.M.; Begg, R.K. A Systematic Review of the Effect of Ageing and Falls History on Minimum Foot Clearance Characteristics during Level Walking. Gait Posture 2010, 32, 429–435. [Google Scholar] [CrossRef]
- Van Wezel, B.M.H.; Ottenhoff, F.A.; Duysens, J. Dynamic Control of Location-Specific Information in Tactile Cutaneous Reflexes from the Foot during Human Walking. J. Neurosci. 1997, 17, 3804–3814. [Google Scholar] [CrossRef]
- Vandervoort, A.A.; Chesworth, B.M.; Cunningham, D.A.; Paterson, D.H.; Rechnitzer, P.A.; Koval, J.J. Age and Sex Effects on Mobility of the Human Ankle. J. Gerontol. 1992, 47, M17–M21. [Google Scholar] [CrossRef]
- Christ, C.B.; Boileau, R.A.; Slaughter, M.H.; Stillman, R.J.; Cameron, J.A.; Massey, B.H. Maximal Voluntary Isometric Force Production Characteristics of Six Muscle Groups in Women Aged 25 to 74 Years. Am. J. Hum. Biol. 1992, 4, 537–545. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Frank, J.S.; Walt, S.E. Biomechanical Walking Pattern Changes in the Fit and Healthy Elderly. Phys. Ther. 1990, 70, 340–347. [Google Scholar] [CrossRef] [PubMed]
- DeVita, P.; Hortobagyi, T. Age Causes a Redistribution of Joint Torques and Powers during Gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Gait, 2nd ed.; University of Waterloo Press: Waterloo, ON, USA, 1991. [Google Scholar]
- Pavol, M.J.; Owings, T.M.; Foley, K.T.; Grabiner, M.D. Mechanisms Leading to a Fall from an Induced Trip in Healthy Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M428–M437. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bogert, A.J.; Pavol, M.J.; Grabiner, M.D. Response Time Is More Important than Walking Speed for the Ability of Older Adults to Avoid a Fall after a Trip. J. Biomech. 2002, 35, 199–205. [Google Scholar] [CrossRef]
- Pijnappels, M.; Reeves, N.D.; Maganaris, C.; van Dieen, J.H. Tripping without falling: Lower limb strength, a limitation for balance recovery and a target for training in the elderly. J. Electromyogr. Kinesiol. 2008, 18, 188–196. [Google Scholar] [CrossRef]
- Siragy, T.; Russo, Y.; Young, W.; Lamb, S.E. Comparison of over-ground and treadmill perturbations for simulation of real-world slips and trips: A systematic review. Gait Posture 2023, 100, 201–209. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodie, R.; Klimstra, M.; Commandeur, D.; Hundza, S. Erosion of Stumble Correction Evoked with Superficial Peroneal Nerve Stimulation in Older Adults during Walking. J. Funct. Morphol. Kinesiol. 2024, 9, 94. https://doi.org/10.3390/jfmk9020094
Brodie R, Klimstra M, Commandeur D, Hundza S. Erosion of Stumble Correction Evoked with Superficial Peroneal Nerve Stimulation in Older Adults during Walking. Journal of Functional Morphology and Kinesiology. 2024; 9(2):94. https://doi.org/10.3390/jfmk9020094
Chicago/Turabian StyleBrodie, Ryan, Marc Klimstra, Drew Commandeur, and Sandra Hundza. 2024. "Erosion of Stumble Correction Evoked with Superficial Peroneal Nerve Stimulation in Older Adults during Walking" Journal of Functional Morphology and Kinesiology 9, no. 2: 94. https://doi.org/10.3390/jfmk9020094
APA StyleBrodie, R., Klimstra, M., Commandeur, D., & Hundza, S. (2024). Erosion of Stumble Correction Evoked with Superficial Peroneal Nerve Stimulation in Older Adults during Walking. Journal of Functional Morphology and Kinesiology, 9(2), 94. https://doi.org/10.3390/jfmk9020094





