Effects of Muscular Fatigue on Position Sense in Two Phases of the Menstrual Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Measuring Instruments
2.4. Preliminary Assessments
2.4.1. Screening Period and Determination of Menstrual Cycle Phases
2.4.2. Determination of Dominant Lower Limb
2.5. Experimental Sessions
2.5.1. Position Sense Assessment
2.5.2. Muscle Fatigue Protocol
2.6. Statistical Analysis
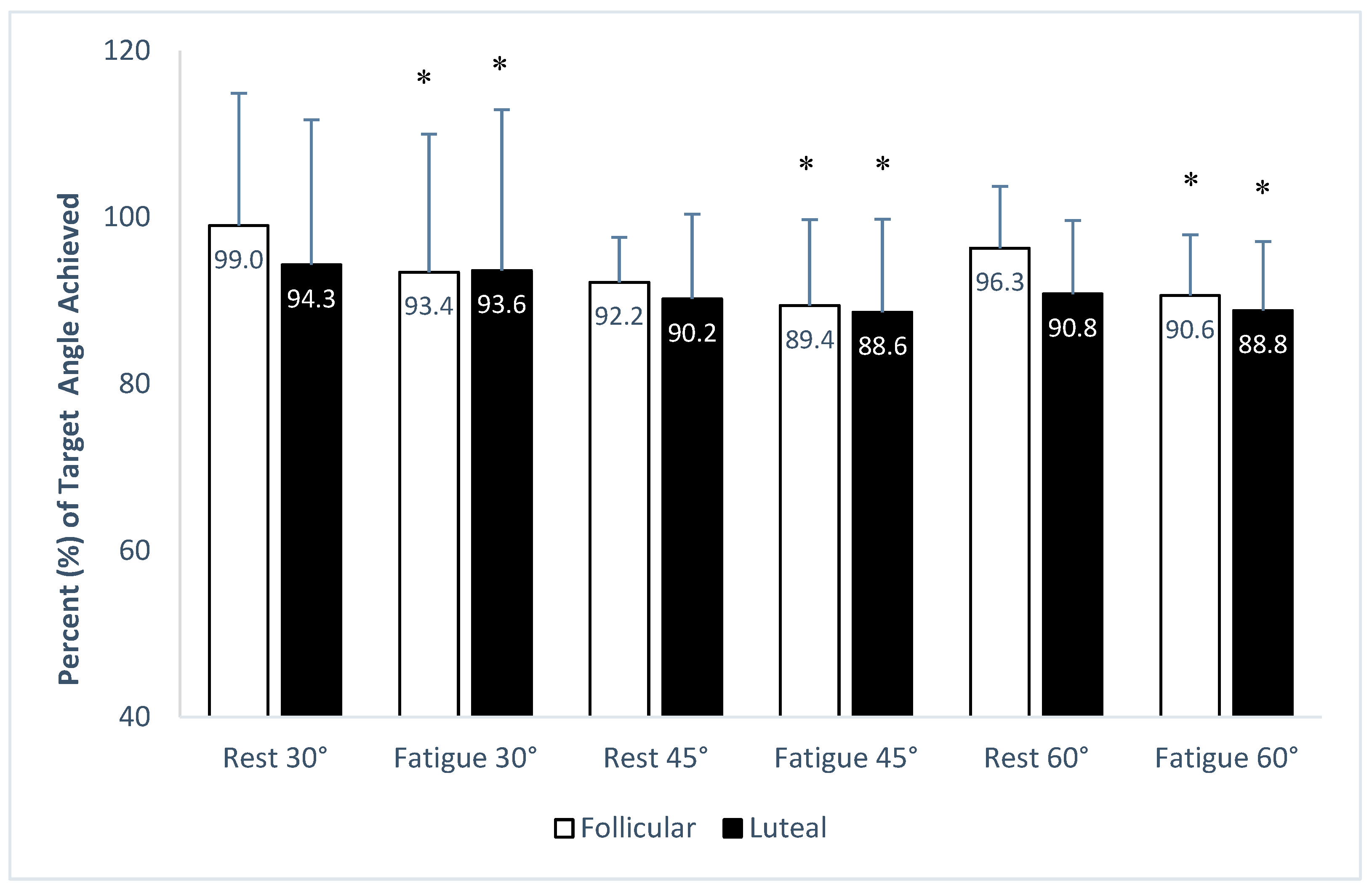
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiemstra, L.A.; Lo, I.K.Y.; Fowler, P.J. Effect of Fatigue on Knee Proprioception: Implications for Dynamic Stabilization. J. Orthop. Sports Phys. Ther. 2001, 31, 598–605. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Babis, G.C.; Soultanis, K.C.; Soucacos, P.N. Functional Neuroanatomy of Proprioception. J. Surg. Orthop. Adv. 2008, 17, 159–164. [Google Scholar] [PubMed]
- Lephart, S.M.; Pincivero, D.M.; Giraido, J.L.; Fu, F.H. The Role of Proprioception in the Management and Rehabilitation of Athletic Injuries. Am. J. Sports Med. 1997, 25, 130–137. [Google Scholar] [CrossRef]
- Fridén, T.; Roberts, D.; Zätterström, R.; Lindstrand, A.; Moritz, U. Proprioception in the Nearly Extended Knee: Measurements of Position and Movement in Healthy Individuals and in Symptomatic Anterior Cruciate Ligament Injured Patients. Knee Surg. Sports Traumatol. Arthrosc. 1996, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Rozzi, S.L.; Lephart, S.M.; Gear, W.S.; Fu, F.H. Knee Joint Laxity and Neuromuscular Characteristics of Male and Female Soccer and Basketball Players. Am. J. Sports Med. 1999, 27, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Nikolaidis, M.G.; Giakas, G.; Jamurtas, A.Z.; Pappas, A.; Koutedakis, Y. The Effect of Eccentric Exercise on Position Sense and Joint Reaction Angle of the Lower Limbs. Muscle Nerve 2007, 35, 496–503. [Google Scholar] [CrossRef]
- Miura, K.; Ishibashi, Y.; Tsuda, E.; Okamura, Y.; Otsuka, H.; Toh, S. The Effect of Local and General Fatigue on Knee Proprioception. Arthrosc. J. Arthrosc. Relat. Surg. 2004, 20, 414–418. [Google Scholar] [CrossRef]
- Riva, D.; Mamo, C.; Fanì, M.; Saccavino, P.; Rocca, F.; Momenté, M.; Fratta, M. Single Stance Stability and Proprioceptive Control in Older Adults Living at Home: Gender and Age Differences. J. Aging Res. 2013, 2013, 561695. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Nixon, J.E.; Reitz, M.; Rindfleish, L.; Tucker, J. Age-Related Changes in Proprioception and Sensation of Joint Position. Acta Orthop. Scand. 1985, 56, 72–74. [Google Scholar] [CrossRef]
- Gokeler, A.; Benjaminse, A.; Hewett, T.E.; Lephart, S.M.; Engebretsen, L.; Ageberg, E.; Engelhardt, M.; Arnold, M.P.; Postema, K.; Otten, E.; et al. Proprioceptive Deficits after ACL Injury: Are They Clinically Relevant? Br. J. Sports Med. 2012, 46, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Garrett, W.E. Mechanisms of Non-Contact ACL Injuries. Br. J. Sports Med. 2007, 41, 47–51. [Google Scholar] [CrossRef]
- Ferretti, A.; Papandrea, P.; Conteduca, F.; Mariani, P.P. Knee Ligament Injuries in Volleyball Players. Am. J. Sports Med. 1992, 20, 203–207. [Google Scholar] [CrossRef]
- Agel, J.; Rockwood, T.; Klossner, D. Collegiate ACL Injury Rates Across 15 Sports: National Collegiate Athletic Association Injury Surveillance System Data Update (2004–2005 Through 2012–2013). Clin. J. Sport Med. 2016, 26, 518–523. [Google Scholar] [CrossRef]
- Şenol, D.; Uçar, C.; Toy, S.; Kısaoğlu, A.; Özbağ, D.; Ersoy, Y.; Yıldız, S. Analysis of the Effects of Hypothalamic-Pituitary-Adrenal Axis Activity in Menstrual Cycle on Ankle Proprioception, Dynamic Balance Scores and Visual-Auditory Reaction Times in Healthy Young Women. J. Musculoskelet. Neuronal. Interact. 2021, 21, 85–92. [Google Scholar] [PubMed]
- Fridén, C.; Hirschberg, A.L.; Saartok, T.; Renström, P. Knee Joint Kinaesthesia and Neuromuscular Coordination during Three Phases of the Menstrual Cycle in Moderately Active Women. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 383–389. [Google Scholar] [CrossRef]
- Shultz, S.J.; Kirk, S.E.; Johnson, M.L.; Sander, T.C.; Perrin, D.H. Relationship between Sex Hormones and Anterior Knee Laxity across the Menstrual Cycle. Med. Sci. Sports Exerc. 2004, 36, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Dieling, S.; Van der Esch, M.; Janssen, T.W.J. Knee Joint Proprioception in Ballet Dancers and Non-Dancers. J. Danc. Med. Sci. 2014, 18, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Chaubet, V.; Maitre, J.; Cormery, B.; Paillard, T. Stimulated and Voluntary Fatiguing Contractions of Quadriceps Femoris Similarly Disturb Postural Control in the Bipedal Stance. Eur. J. Appl. Physiol. 2012, 112, 1881–1887. [Google Scholar] [CrossRef]
- Pincivero, D.M.; Bachmeier, B.; Coelho, A.J. The Effects of Joint Angle and Reliability on Knee Proprioception. Med. Sci. Sports Exerc. 2001, 33, 1708–1712. [Google Scholar] [CrossRef]
- Klein, C.J.D.; Landry, S.C.; Lattimer, L.J. Sex-Based Differences in Lower Extremity Kinematics During Dynamic Jump Landing Tasks After Neuromuscular Fatigue of the Hip Extensors and Knee Flexors. Orthop. J. Sports Med. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Ageberg, E.; Andersson, G.; Fridén, T. Clinical Measurements of Proprioception, Muscle Strength and Laxity in Relation to Function in the ACL-Injured Knee. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 9–16. [Google Scholar] [CrossRef]
- Brozek, J.; Henschel, A. Techniques for Measuring Body Composition; National Academy of Sciences—National Research Council: Washington, DC, USA, 1961; pp. 100–102. [Google Scholar]
- Paschalis, V.; Nikolaidis, M.G.; Giakas, G.; Jamurtas, A.Z.; Owolabi, E.O.; Koutedakis, Y. Position Sense and Reaction Angle after Eccentric Exercise: The Repeated Bout Effect. Eur. J. Appl. Physiol. 2008, 103, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Manou, V.; Arseniou, P.; Gerodimos, V.; Kellis, S. Test-Retest Reliability of an Isokinetic Muscle Endurance Test. Isokinet. Exerc. Sci. 2002, 10, 177–181. [Google Scholar] [CrossRef]
- Baker, F.C.; Siboza, F.; Fuller, A. Temperature Regulation in Women: Effects of the Menstrual Cycle. Temperature 2020, 7, 226–262. [Google Scholar] [CrossRef]
- Jameson, J.L.; DeGroot, L.J. Endocrinology (Adult and Pediatric); Elsevier: Philadelphia, PA, USA, 2015; p. 2298. [Google Scholar]
- Gear, W.S. Effect of Different Levels of Localized Muscle Fatigue on Knee Position Sense. J. Sports Sci. Med. 2011, 10, 725–730. [Google Scholar]
- Carrasco, L.; Espinar, J.; Carbonell, F.J.; Martínez-Díaz, I.C. Fatiga Local y General: Efectos Sobre La Propiocepción de Rodilla En Futbolistas. Rev. Int. Med. Cienc. Act. Física Deporte 2021, 21, 683–698. [Google Scholar] [CrossRef]
- Ju, Y.Y.; Wang, C.W.; Cheng, H.Y.K. Effects of Active Fatiguing Movement versus Passive Repetitive Movement on Knee Proprioception. Clin. Biomech. 2010, 25, 708–712. [Google Scholar] [CrossRef]
- Changela, P.K.; Selvamani, K. A Study to Evaluate the Effect of Fatigue on Knee Joint Proprioception and Balance in Healthy Individuals. Med. Sport. 2012, 3, 1851–1857. [Google Scholar]
- Cengiz, D.U.; Colak, C.S.; Koca, U.H. Comparison of Functional Vestibulo-Ocular Reflex in Follicular and Luteal Phase in Young Girls. J. Int. Adv. Otol. 2023, 19, 517–522. [Google Scholar] [CrossRef]
- Figueiredo, D.G.B.; Rezende, C.M.T.; Santos, A.N.; Andrade, O.J.M. Mapping Changes in women’s visual functions during the menstrual cycle: Narrative review. Sao Paulo Med. J. 2021, 139, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Daniusevičiūtė, L.; Linonis, V.; Barsienė, L. The Effect of Increased Female Sex Hormone Concentration on Movement Proprioception. Balt. J. Sport Health Sci. 2012, 3, 26–32. [Google Scholar] [CrossRef]
- Aydoğ, S.T.; Hasçelik, Z.; Demirel, H.A.; Tetik, O.; Aydoğ, E.; Doral, M.N. The Effects of Menstrual Cycle on the Knee Joint Position Sense: Preliminary Study. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Hesar, N.G.Z.; Calders, P.; Thijs, Y.; Roosen, P.; Witvrouw, E. The Influence of Menstrual Cycle on Ankle Proprioception. Isokinet. Exerc. Sci. 2008, 16, 119–123. [Google Scholar] [CrossRef]
- Fouladi, R.; Rajabi, R.; Naseri, N.; Pourkazemi, F.; Geranmayeh, M. Menstrual Cycle and Knee Joint Position Sense in Healthy Female Athletes. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Williams, N.I.; Olmsted-Kramer, L.C.; Leidy, H.J.; Putukian, M. Neuromuscular Performance and Knee Laxity Do Not Change across the Menstrual Cycle in Female Athletes. Knee Surg. Sports Traumatol. Arthr. 2006, 14, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Ochi, M.; Uchio, Y.; Iwasa, J.; Ryoke, K.; Kuriwaka, M. Mechanoreceptors in the Anterior Cruciate Ligament Contribute to the Joint Position Sense. Acta Orthop. Scand. 2002, 73, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Stefanyshyn, D.J.; Ramage, B.; Hart, D.A.; Ronsky, J.L. Alterations in Knee Joint Laxity During the Menstrual Cycle in Healthy Women Leads to Increases in Joint Loads During Selected Athletic Movements. Am. J. Sports Med. 2009, 37, 1169–1177. [Google Scholar] [CrossRef]
- Allison, K.F.; Abt, J.P.; Beals, K.; Nagle, E.F.; Lovalekar, M.T.; Lephart, S.M.; Sell, T.C. Aerobic Capacity and Isometric Knee Flexion Strength Fatigability Are Related to Knee Kinesthesia in Physically Active Women. Isokinet. Exerc. Sci. 2016, 24, 357–365. [Google Scholar] [CrossRef]
- Hewett, T.E. Neuromuscular and Hormonal Factors Associated With Knee Injuries in Female Athletes: Strategies for Intervention. Sports Med. 2000, 29, 313–327. [Google Scholar] [CrossRef]
- Wang, L.I. The Lower Extremity Biomechanics of Single- and Double-Leg Stop-Jump Tasks. J. Sports Sci. Med. 2011, 10, 151–156. [Google Scholar] [PubMed]
- Lessi, G.C.; Dos Santos, A.F.; Batista, L.F.; De Oliveira, G.C.; Serrão, F.V. Effects of Fatigue on Lower Limb, Pelvis and Trunk Kinematics and Muscle Activation: Gender Differences. J. Electromyogr. Kinesiol. 2017, 32, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Schmalenberger, K.M.; Tauseef, H.A.; Barone, J.C.; Owens, S.A.; Lieberman, L.; Jarczok, M.N.; Girdler, S.S.; Kiesner, J.; Ditzen, B.; Eisenlohr-Moul, T.A. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology 2021, 123, 104895. [Google Scholar] [CrossRef] [PubMed]
| Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|
| Age (years) | 19 | 30 | 23.80 | 3.17 |
| Weight (kg) | 51 | 76 | 60.22 | 6.31 |
| Height (m) | 1.49 | 1.83 | 1.64 | 0.85 |
| BMI (kg/m2) | 22.69 | 22.97 | 22.83 | 0.28 |
| Target Angles | Menstrual Phases | Conditions | |
|---|---|---|---|
| Rest | Fatigue | ||
| 30° | Follicular | −0.34 ± 4.82 | −2.00 ± 4.98 * |
| Luteal | −2.08 ± 5.36 | −0.597 ± 6.09 * | |
| 45° | Follicular | −3.57 ± 2.42 # | −4.76 ± 4.61 *# |
| Luteal | −4.43 ± 4.59 # | −5.15 ± 5.03 *# | |
| 60° | Follicular | −2.24 ± 4.41 # | −5.65 ± 4.39 *# |
| Luteal | −5.55 ± 5.29 # | −6.73 ± 4.99 *# | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roditi, E.-E.; Tsatalas, T.; Sakkas, G.K.; Koutedakis, Y.; Giakas, G.; Karatzaferi, C. Effects of Muscular Fatigue on Position Sense in Two Phases of the Menstrual Cycle. J. Funct. Morphol. Kinesiol. 2024, 9, 115. https://doi.org/10.3390/jfmk9030115
Roditi E-E, Tsatalas T, Sakkas GK, Koutedakis Y, Giakas G, Karatzaferi C. Effects of Muscular Fatigue on Position Sense in Two Phases of the Menstrual Cycle. Journal of Functional Morphology and Kinesiology. 2024; 9(3):115. https://doi.org/10.3390/jfmk9030115
Chicago/Turabian StyleRoditi, Elmina-Eleftheria, Themistoklis Tsatalas, Giorgos K. Sakkas, Yiannis Koutedakis, Giannis Giakas, and Christina Karatzaferi. 2024. "Effects of Muscular Fatigue on Position Sense in Two Phases of the Menstrual Cycle" Journal of Functional Morphology and Kinesiology 9, no. 3: 115. https://doi.org/10.3390/jfmk9030115







