Active Commuting as a Factor of Cardiovascular Disease Prevention: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. SPIDER
2.2. Eligibility Criteria
2.3. Outcome Measures
2.4. Data Sources and Search Strategy
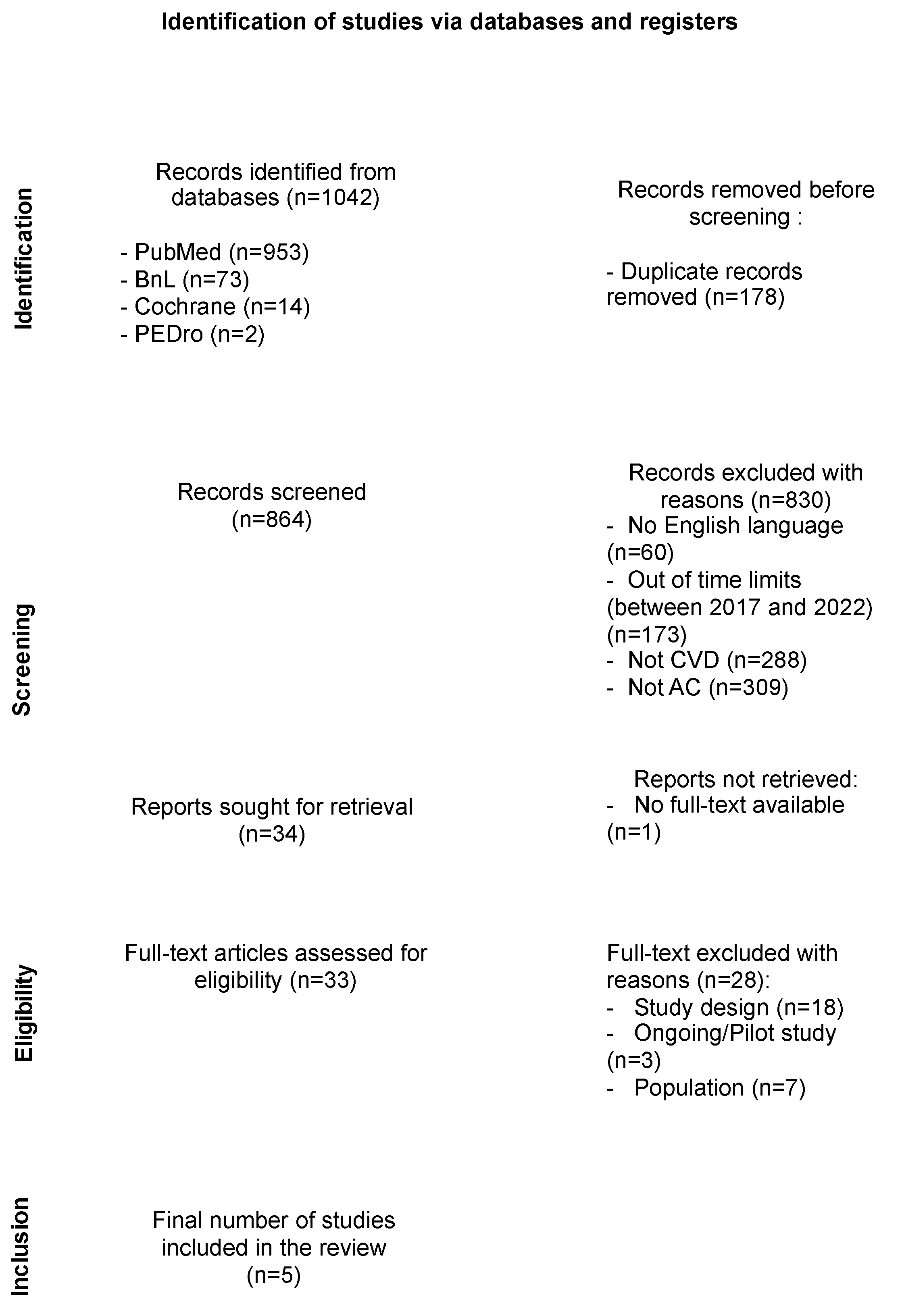
2.5. Study Selection
2.6. Quality Assessment
2.7. Data Extraction
2.8. Statistical Analysis
2.9. Certainty of Evidence
3. Results
3.1. Study Characteristics
3.2. Methodological Quality and Risk of Bias
- Reporting bias: all five articles were classified with a low-level bias in this domain.
3.3. Certainty of Evidence
3.4. Effect of AC on CVD
4. Discussion
4.1. Summary of Main Findings
4.2. Strengths and Limitations
4.3. Comparisons across Studies
4.4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases. Fact Sheet #317. 2017. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 22 December 2023).
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary Prevention of Cardiovascular Disease: A Review of Contemporary Guidance and Literature. JRSM Cardiovasc Dis. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Taub, P.R.; Epstein, E.; Michos, E.D.; Ferraro, R.A.; Bailey, A.L. Ten Things to Know About Ten Cardiovascular Disease Risk Factors. Am. J. Prev. Cardiol. 2021, 5, 100149–100165. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H. Overview of Primary Prevention of Cardiovascular Disease. UpToDate. 2021. Available online: https://www.uptodate.com/contents/overview-of-primary-prevention-of-cardiovascular-disease (accessed on 22 December 2023).
- Beevers, D.G. The Atlas of Heart Disease and Stroke. J. Hum. Hypertens. 2005, 19, 505. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. Available online: https://www.isca-web.org/files/Global_Recommendation_on_physical_activity_for_health_WHO.pdf (accessed on 22 December 2023).
- Matthews, C.E. Minimizing Risk Associated with Sedentary Behavior: Should We Focus on Physical Activity, Sitting, or Both? J. Am. Coll. Cardiol. 2019, 73, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, E.; Gale, J.; Bauman, A.; Ekelund, U.; Hamer, M.; Ding, D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019, 73, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Pucher, J.; Buehler, R.; Thompson, D.L.; Crouter, S.E. Walking, Cycling, and Obesity Rates in Europe, North America and Australia. J. Phys. Act. Health. 2008, 5, 795–814. [Google Scholar] [CrossRef]
- Flint, E.; Cummins, S.; Sacker, A. Associations Between Active Commuting, Body Fat, and Body Mass Index: Population-Based, Cross-Sectional Study in the United Kingdom. BMJ 2014, 349, g4887. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, D.K.; Goodman, A.; Ogilvie, D. Associations Between Active Commuting and Physical and Mental Well-Being. Prev. Med. 2013, 57, 135–139. [Google Scholar] [CrossRef]
- Laverty, A.A.; Palladino, R.; Lee, J.T.; Millett, C. Associations Between Active Travel and Weight, Blood Pressure and Diabetes in Six Middle-Income Countries: A Cross-Sectional Study in Older Adults. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Millett, C.; Agrawal, S.; Sullivan, R.; Vaz, M.; Kurpad, A.; Bharathi, A.V.; Prabhakaran, D.; Reddy, K.S.; Kinra, S.; Smith, G.D.; et al. Associations Between Active Travel to Work and Overweight, Hypertension, and Diabetes in India: A Cross-Sectional Study. PLoS Med. 2013, 10, 1–11. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Active Commuting and Cardiovascular Risk: A Meta-Analytic Review. Prev. Med. 2008, 46, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Tuomilehto, J.; Borodulin, K.; Jousilahti, P. The Joint Associations of Occupational, Commuting, and Leisure-Time Physical Activity, and the Framingham Risk Score on the 10-Year Risk of Coronary Heart Disease. Eur. Heart J. 2007, 28, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Laverty, A.A.; Mindell, J.S.; Webb, E.A.; Millett, C. Active Travel to Work and Cardiovascular Risk Factors in the United Kingdom. Am. J. Prev. Med. 2013, 45, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Simon, C.; Evans, A.; Ferrières, J.; Montaye, M.; Ducimetière, P.; Arveiler, D. Physical Activity and Coronary Event Incidence in Northern Ireland and France: The Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2002, 105, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.B.; Schnohr, P.; Schroll, M.; Hein, H.O. All-Cause Mortality Associated with Physical Activity During Leisure Time, Work, Sports, and Cycling to Work. Arch. Intern. Med. 2000, 160, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Jurj, A.L.; Shu, X.O.; Li, H.L.; Yang, G.; Li, Q.; Gao, Y.-T.; Zheng, W. Influence of Exercise, Walking, Cycling, and Overall Nonexercise Physical Activity on Mortality in Chinese Women. Am. J. Epidemiol. 2007, 165, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- de Geus, B.; van Hoof, E.; Aerts, I.; Meeusen, R. Cycling to Work: Influence on Indexes of Health in Untrained Men and Women in Flanders. Scand. J. Med. Sci. Sports 2008, 18, 498–510. [Google Scholar] [CrossRef] [PubMed]
- de Geus, B.; Joncheere, J.; Meeusen, R. Commuter Cycling: Effect on Physical Performance in Untrained Men and Women in Flanders: Minimum Dose to Improve Indexes of Fitness. Scand. J. Med. Sci. Sports 2009, 19, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Gram, A.S.; Bladbjerg, E.M.; Quist, J.S.; Petersen, M.B.; Rosenkilde, M.; Stallknecht, B. Anti-Inflammatory Effects of Active Commuting and Leisure Time Exercise in Overweight and Obese Women and Men: A Randomized Controlled Trial. Atherosclerosis 2017, 265, 318–324. [Google Scholar] [CrossRef]
- Hemmingsson, E.; Uddén, J.; Neovius, M.; Ekelund, U.; Rössner, S. Increased Physical Activity in Abdominally Obese Women Through Support for Changed Commuting Habits: A Randomized Clinical Trial. Int. J. Obes. 2009, 33, 645–652. [Google Scholar] [CrossRef]
- Hendriksen, I.J.M.; Zuiderveld, B.; Kemper, H.C.G.; Bezemer, P.D. Effect of Commuter Cycling on Physical Performance of Male and Female Employees. Med. Sci. Sports Exerc. 2000, 32, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Møller, N.C.; Østergaard, L.; Gade, J.R.; Nielsen, J.L.; Andersen, L.B. The Effect on Cardiorespiratory Fitness After an 8-Week Period of Commuter Cycling—A Randomized Controlled Study in Adults. Prev. Med. 2011, 53, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Oja, P.; Mänttäri, A.; Heinonen, A.; Kukkonen-Harjula, K.; Laukkanen, R.; Pasanen, M.; Vuori, P.I. Physiological Effects of Walking and Cycling to Work. Scand. J. Med. Sci. Sports 1991, 1, 151–157. [Google Scholar] [CrossRef]
- Quist, J.S.; Rosenkilde, M.; Petersen, M.B.; Gram, A.S.; Sjödin, A.; Stallknecht, B. Effects of Active Commuting and Leisure-Time Exercise on Fat Loss in Women and Men with Overweight and Obesity: A Randomized Controlled Trial. Int. J. Obes. 2018, 42, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.D.; Ogilvie, D. Motivations for Active Commuting: A Qualitative Investigation of the Period of Home or Work Relocation. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kotseva, K.; de Backer, G.; de Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Lifestyle and Impact on Cardiovascular Risk Factor Control in Coronary Patients Across 27 Countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V Registry. Eur. J. Prev. Cardiol. 2019, 26, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Samitz, G.; Egger, M.; Zwahlen, M. Domains of Physical Activity and All-Cause Mortality: Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. Int. J. Epidemiol. 2011, 40, 1382–1400. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, J.; Franco, O.H.; Orsini, N.; Roberts, I. Non-Vigorous Physical Activity and All-Cause Mortality: Systematic Review and Meta-Analysis of Cohort Studies. Int. J. Epidemiol. 2011, 40, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Oja, P.; Titze, S.; Bauman, A.; de Geus, B.; Krenn, P.; Reger-Nash, B.; Kohlberger, T. Health Benefits of Cycling: A Systematic Review. Scand. J. Med. Sci. Sports 2011, 21, 496–509. [Google Scholar] [CrossRef]
- Saunders, L.E.; Green, J.M.; Petticrew, M.P.; Steinbach, R.; Roberts, H. What Are the Health Benefits of Active Travel? A Systematic Review of Trials and Cohort Studies. PLoS ONE 2013, 8, e69912. [Google Scholar] [CrossRef]
- Autenrieth, C.S.; Baumert, J.; Baumeister, S.E.; Fischer, B.; Peters, A.; Döring, A.; Thorand, B. Association Between Domains of Physical Activity and All-Cause, Cardiovascular and Cancer Mortality. Eur. J. Epidemiol. 2011, 26, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.A.; Lyall, D.M.; Welsh, P.; Anderson, J.; Steell, L.; Guo, Y.; Maldonado, R.; Mackay, D.F.; Pell, J.P.; Sattar, N.; et al. Association Between Active Commuting and Incident Cardiovascular Disease, Cancer, and Mortality: Prospective Cohort Study. BMJ 2017, 357, j1456. [Google Scholar] [CrossRef] [PubMed]
- Wanner, M.; Götschi, T.; Martin-Diener, E.; Kahlmeier, S.; Martin, B.W. Active Transport, Physical Activity, and Body Weight in Adults: A Systematic Review. Am. J. Prev. Med. 2012, 42, 493–502. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Brozek, J. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, 336–341. [Google Scholar] [CrossRef]
- Cooke, A.; Smith, D.; Booth, A. Beyond PICO: The SPIDER Tool for Qualitative Evidence Synthesis. Qual. Health Res. 2012, 22, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Peterman, J.E.; Bassett, D.R.; Holmes Finch, W.; Harber, M.P.; Whaley, M.H.; Fleenor, B.S.; Kaminsky, L.A. Associations Between Active Commuting and Cardiovascular Disease in the United States. J. Phys. Act. Health 2021, 18, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Bagias, C.; Sukumar, N.; Weldeselassie, Y.; Oyebode, O.; Saravanan, P. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Int. J. Environ. Res. Public Health 2021, 18, 1897. [Google Scholar] [CrossRef] [PubMed]
- EPHPP. Quality Assessment Tool for Quantitative Studies. Effective Public Health Practice Project; McMaster University: Toronto, ON, Canada, 2010. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating the Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924. [Google Scholar] [CrossRef] [PubMed]
- Blond, K.; Jensen, M.K.; Rasmussen, M.G.; Overvad, K.; Tjønneland, A.; Østergaard, L.; Grøntved, A. Prospective Study of Bicycling and Risk of Coronary Heart Disease in Danish Men and Women. Circulation 2016, 134, 1409–1411. [Google Scholar] [CrossRef]
- Eriksson, J.S.; Ekblom, B.; Kallings, L.V.; Hemmingsson, E.; Andersson, G.; Wallin, P.; Ekblom, Ö.; Ekblom-Bak, E. Active Commuting in Swedish Workers Between 1998 and 2015—Trends, Characteristics, and Cardiovascular Disease Risk. Scand. J. Med. Sci. Sports 2020, 30, 370–379. [Google Scholar] [CrossRef]
- Kaiser, M.; Bauer, J.M.; Otterbach, S.; Reisch, L.A.; Sousa-Poza, A. The Association Between Commuting and Cardiovascular Disease: A Biomarker-Based Analysis of Cross-Sectional Cohort Data from the UK Biobank. Prev. Med. 2023, 72, 107521. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Davis, R.E. Effects of Individual, Combined, and Isolated Physical Activity Behaviours on All-Cause Mortality and CVD-Specific Mortality: Prospective Cohort Study Among US Adults. Physiol. Behav. 2015, 151, 355–359. [Google Scholar] [CrossRef]
- Bauman, A.E.; Grunseit, A.C.; Rangul, V.; Heitmann, B.L. Physical Activity, Obesity and Mortality: Does the Pattern of Physical Activity Have Stronger Epidemiological Associations? BMC Public Health 2017, 17, 788. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook—Introduction to GRADE Handbook: Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach; Updated October 2013; The GRADE Working Group. 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 22 December 2023).
- Fan, M.; Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, S.; Yang, L.; Chen, Y.; Huang, Y.; Chen, B.; et al. Association Between Active Commuting and Incident Cardiovascular Diseases in Chinese: A Prospective Cohort Study. J. Am. Heart Assoc. 2019, 8, e012556. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Patsopoulos, N.A.; Rothstein, H.R. Reasons or Excuses for Avoiding Meta-Analysis in Forest Plots. BMJ 2008, 336, 1413–1415. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M. Does Physical Activity Attenuate, or Even Eliminate, the Detrimental Association of Sitting Time with Mortality? A Harmonized Meta-Analysis of Data from More Than 1 Million Men and Women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Oja, P.; Kelly, P.; Pedisic, Z.; Titze, S.; Bauman, A.; Foster, C.; Hamer, H.; Hillsdon, M.; Stamatakis, E. Associations of Specific Types of Sports and Exercise with All-Cause and Cardiovascular-Disease Mortality: A Cohort Study of 80,306 British Adults. Br. J. Sports Med. 2017, 51, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Wanner, M.; Tarnutzer, S.; Martin-Diener, E.; Braun, J.; Rohrmann, S.; Bopp, M.; Faeh, D.; Cohort, S.N. Impact of Different Domains of Physical Activity on Cause-Specific Mortality: A Longitudinal Study. Prev. Med. 2014, 62, 89–95. [Google Scholar] [CrossRef]
- Johansson, C.; Lövenheim, B.; Schantz, P.; Wahlgren, L.; Almström, P.; Markstedt, A.; Strömgren, M.; Forsberg, B.; Sommar, J.N. Impacts on Air Pollution and Health by Changing Commuting from Car to Bicycle. Sci. Total Environ. 2017, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.; Rojas-Rueda, D.; Cole-Hunter, T.; de Nazelle, A.; Dons, E.; Gerike, R.; Götschi, T.; Panis, L.T.; Kahlmeier, S.; Nieuwenhuijsen, M. Health Impact Assessment of Active Transportation: A Systematic Review. Prev. Med. 2015, 76, 103–114. [Google Scholar] [CrossRef]
- Fontaras, G.; Zacharof, N.G.; Ciuffo, B. Fuel Consumption and CO2 Emissions from Passenger Cars in Europe—Laboratory Versus Real-World Emissions. Prog. Energy Combust. Sci. 2017, 60, 97–131. [Google Scholar] [CrossRef]
- Sadik-Khan, J.; Solomonow, S. Improving Public Health by Making Cities Friendly to Walking and Biking: Safer, More Active Transportation Starts with the Street. JAMA Intern. Med. 2017, 177, 613–614. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Macchi, C.; Sofi, F. Active Commuting and Multiple Health Outcomes: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 437–452. [Google Scholar] [CrossRef]
- Flint, E.; Webb, E.; Cummins, S. Change in Commute Mode and Body-Mass Index: Prospective, Longitudinal Evidence from UK Biobank. Lancet Public Health 2016, 1, e46–e55. [Google Scholar] [CrossRef]
- Shuttleworth, I.; Gould, M. Distance Between Home and Work: A Multilevel Analysis of Individual Workers, Neighbourhoods, and Employment Sites in Northern Ireland. Environ. Plan. A 2010, 42, 1221–1238. [Google Scholar] [CrossRef]
- Sun, D.; Liu, C.; Ding, Y.; Yu, C.; Guo, Y.; Sun, D.; Pang, Y.; Pei, P.; Du, H.; Yang, L.; et al. Long-Term Exposure to Ambient PM2.5, Active Commuting, and Farming Activity and Cardiovascular Disease Risk in Adults in China: A Prospective Cohort Study. Lancet Planet Health 2023, 7, e304–e312. [Google Scholar] [CrossRef] [PubMed]

| Primary Terms | Secondary Terms |
|---|---|
|
|
| First Author (Year) | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bauman et al., (2017) [43] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | X | 0 | 1 | Good |
| Blond et al., (2016) [44] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | X | 0 | 1 | Good |
| Eriksson et al., (2020) [45] | 1 | 1 | X | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | X | 1 | 1 | Good |
| Fan et al., (2019) [46] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Loprinzi & Davis, (2015) [47] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | X | 1 | 1 | Good |
| Study Author | Country | Date Source | Study Design | Sample Size (n) | Age Range (Years) | Follow Up (Years) | Exposure | Exposure Measurement | Other PA Domains | Analysis Method | Outcome Definition | Events (n) | Controlling or Adjustment Variables |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bauman et al., (2017) [43] | Denmark | Danish MONICA study | Cross-sectional | 2.829 | 30–61 | 19 | Walking, bicycling to work | Self-reported | Leisure time PA, sport participation | Cox proportional hazards regression | All-cause mortality | 668/2829 | Age, sex, BMI, educational level, occupation, housing, waist and hip circumference |
| CHD mortality | 290/2829 | ||||||||||||
| CVD mortality | 125/2829 | ||||||||||||
| Blond et al., (2016) [44] | Denmark | Diet, Cancer and Health study | Prospective Cohort | 53.723 | 50–65 | 20 | bicycling to work | Self-reported | Leisure time cycling, and other PA | Cox proportional hazards regression | CHD event | 2892/53.723 | BMI, educational level, hypertension medication, hypercholesterolemia medication, self-reported diabetes medication, diet, alcohol, smoking |
| Eriksson et al., (2020) [45] | Sweden | Health Profile Institute | Prospective Cohort | 318.309 | 18–74 | 17 | Walking, bicycling, public transportation or car | Self-reported | Leisure time exercise, physical work situation | Cox proportional hazards regression | CVD events (fatal or non-fatal myocardial infarction, angina pectoris, or ischemic stroke) | 5714/318.309 | Age, sex, BMI, VO2max, beta-blockers, educational level, diet, smoking, perceived overall health |
| Fan et al., (2019) [46] | China | CKB data | Prospective cohort | 104.170 | 35–74 | 10 | Walking, bicycling | Self-reported | Leisure sedentary time, occupational commuting household | Cox proportional hazards regression | Ischemic heart disease | 5374/104.170 | Age, sex, BMI, educational level, marital status, household income, occupation, alcohol, smoking, red meat intake, fresh fruits and vegetables intake, hypertension, diabetes mellitus, family histories of heart attack or stroke |
| Ischemic stroke | 664/104.170 | ||||||||||||
| Hemorrhagic stroke | 4834/104.170 | ||||||||||||
| Loprinzi & Davis, (2015) [47] | U.S.A. | NHANES cycles | Prospective cohort | 12.321 | 20–85 | 5 | Walking, bicycling to work | Self-reported | Moderate-to-vigorous intensity aerobic PA, muscular strength activities | Cox proportional hazards regression | All-cause mortality | 654/12.321 | Age, sex, BMI, ethnicity, educational level, smoking, C-reactive protein |
| CVD mortality | 231/12.321 |
| Number of Studies (Subjects) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Grades of Recommendation |
|---|---|---|---|---|---|---|
| 5 (n = 491,352) | Low | Low | High | High | High | Strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, C.; Belgacem, S.; Paillet, M.; de Abreu, R.M.; de Araujo, F.X.; Meroni, R.; Corbellini, C. Active Commuting as a Factor of Cardiovascular Disease Prevention: A Systematic Review with Meta-Analysis. J. Funct. Morphol. Kinesiol. 2024, 9, 125. https://doi.org/10.3390/jfmk9030125
Baran C, Belgacem S, Paillet M, de Abreu RM, de Araujo FX, Meroni R, Corbellini C. Active Commuting as a Factor of Cardiovascular Disease Prevention: A Systematic Review with Meta-Analysis. Journal of Functional Morphology and Kinesiology. 2024; 9(3):125. https://doi.org/10.3390/jfmk9030125
Chicago/Turabian StyleBaran, Claudia, Shanice Belgacem, Mathilde Paillet, Raphael Martins de Abreu, Francisco Xavier de Araujo, Roberto Meroni, and Camilo Corbellini. 2024. "Active Commuting as a Factor of Cardiovascular Disease Prevention: A Systematic Review with Meta-Analysis" Journal of Functional Morphology and Kinesiology 9, no. 3: 125. https://doi.org/10.3390/jfmk9030125
APA StyleBaran, C., Belgacem, S., Paillet, M., de Abreu, R. M., de Araujo, F. X., Meroni, R., & Corbellini, C. (2024). Active Commuting as a Factor of Cardiovascular Disease Prevention: A Systematic Review with Meta-Analysis. Journal of Functional Morphology and Kinesiology, 9(3), 125. https://doi.org/10.3390/jfmk9030125








