Abstract
Elective mandibular surgical osteotomies are commonly used to correct craniofacial discrepancies. Since the modifications proposed by Obwegeser, Dal Pont, and Hunsuck, no effective variations have been proposed to improve the biomechanical results of these mandibular osteotomies. With technological developments and the use of three-dimensional images from CT scans of patients, much has been done to plan and predict outcomes with greater precision and control. To date, 3D imaging and additive manufacturing technologies have not been used to their full potential to create innovative mandibular osteotomies. The use of 3D digital images obtained from CT scans as DICOM files, which were then converted to STL files, proved to be an efficient method of developing an innovative mandibular ramus beveled osteotomy technique. The new mandibular osteotomy is designed to reduce the likelihood of vasculo-nervous damage to the mandible, reduce the time and ease of surgery, and reduce post-operative complications. The proposed osteotomy does not affect traditional osteotomies. Anatomical structures such as the inferior alveolar nerve and intraoral surgical access were preserved and maintained, respectively. The results obtained from the digital images were validated on an additively manufactured 3D synthetic bone model.
1. Introduction
This study describes the feasibility of developing a novel mandibular osteotomy through 3D digital planning, 3D model validation, and patient-matched surgical guide fabrication.
Muscle biomechanics is the study of the internal and external forces that act on the human body to produce movement. It examines how muscles, bones, tendons, and ligaments work together to produce function. Muscles can only pull and come in antagonistic pairs. When one muscle in a pair contracts, the other relaxes, and vice versa. The function of muscles depends on their intrinsic properties and extrinsic arrangement [1].
Contraction refers to the generation of tension within a muscle fiber through actin and myosin cross-bridge cycling. The sarcoplasm of a muscle can lengthens, shorten, or remain the same length under tension. The names of the contractions are based on how the length of the sarcoplasm changes during this tension [2].
Isokinetic contractions occur at a constant rate. Isotonic contractions involve constant tension as the muscle changes length and can be either concentric (shortening the muscle) or eccentric (lengthening the muscle). Isometric contractions involve no change in muscle length [2,3].
The mandible has seven main muscles: the buccinator, which assists in chewing and originates from the alveolar process of the mandible; the mylohyoid, which is the primary muscle of the mouth floor; the superior pharyngeal constrictor, which attaches to the mylohyoid and plays a crucial role in swallowing [3].
The motor fibers of the mandibular branch of the trigeminal nerve (CN V3) innervate all masticatory muscles, while the main arterial supply comes from branches of the maxillary artery. Muscle is essential for developing bone strength, providing mechanical protection, and preserving or repairing skeletal tissue [1].
Bone undergoes adaptive processes in response to habitual loading, regulating its structure based on various components of its loading regime and mechanical environment [1,4,5].
The interaction between stress and strain provides insight into the mechanical behavior of the properties of bone material as it deforms under load [1].
Tooth loss affects the fine proprioceptive control of jaw function and influences the precision of occlusal load application. This is due to the removal of intra-dental and periodontal mechanoreception. Osseoperception, on the other hand, depends on central influences from corollary discharge from cortico-motor commands to jaw muscles and contributions from peripheral mechanoreceptors in orofacial and temporomandibular tissues [3,6].
The plasticity of neuromotor mechanisms is recognized in the processing of central influences to accommodate the loss of dental and periodontal inputs [6].
Proprioceptive signals from mechanoreceptors in the joints, muscles, tendons, and skin are crucial for the neural control of movement. The absence of proprioceptive afferents can affect muscle tone control and bone formation, disrupt postural reflexes, and severely impair the spatial and temporal aspects of voluntary movement. There is initial evidence suggesting that proprioceptive training induces cortical reorganization, which reinforces the notion that proprioceptive training is a viable method for improving sensorimotor function [7].
When performing an osteotomy on the mandible, the muscles separated by the posterior and anterior fractured bone segments exert a vectorial force with direction and directionality [3,8].
Pathological fractures can be classified as either favorable or unfavorable to fracture reduction and immobilization treatment. A fracture is considered favorable if the muscle vector force brings the fractured bone segments closer together and unfavorable if it separates the fragments [8].
During elective osteotomy for maxillofacial correction with mandibular osteotomy, it is essential to monitor muscle action. This is necessary not only to maintain stability after immobilization of the surgical fracture but also to ensure a controlled outcome during the period of bone healing and repair [9].
Based on the biomechanics, physiology, and anatomy of the mandible, the ideal mandibular osteotomy for treating craniofacial discrepancies can be determined. The ramus and angle of the mandible bilaterally have been the most commonly used region for osteotomy in elective mandibular surgery throughout history [7,8,9].
The masseter, temporalis, medial, and lateral pterygoid muscles are directly associated with the posterior mandibular fragment in a bilateral sagittal split osteotomy. They exert unnecessary force that can cause unfavorable displacement of the sagittal split osteotomy [3,5].
The other muscles—the mylohyoid, buccinator, and superior pharyngeal constrictor—are directly related to the displacement of the anterior segment of the mandibular osteotomy performed at the angle of the mandible [9].
This work presents an innovative osteotomy technique developed using three-dimensional (3D) technologies, digital imaging, and additive manufacturing of a human mandible obtained from a computed tomography scan. The technique aims to preserve the inferior alveolar vasculo-nervous bundle and the dento-alveolar boundaries while maintaining the integrity of the teeth. To achieve this, the position of the mandibular canal was assessed using the obtained image.
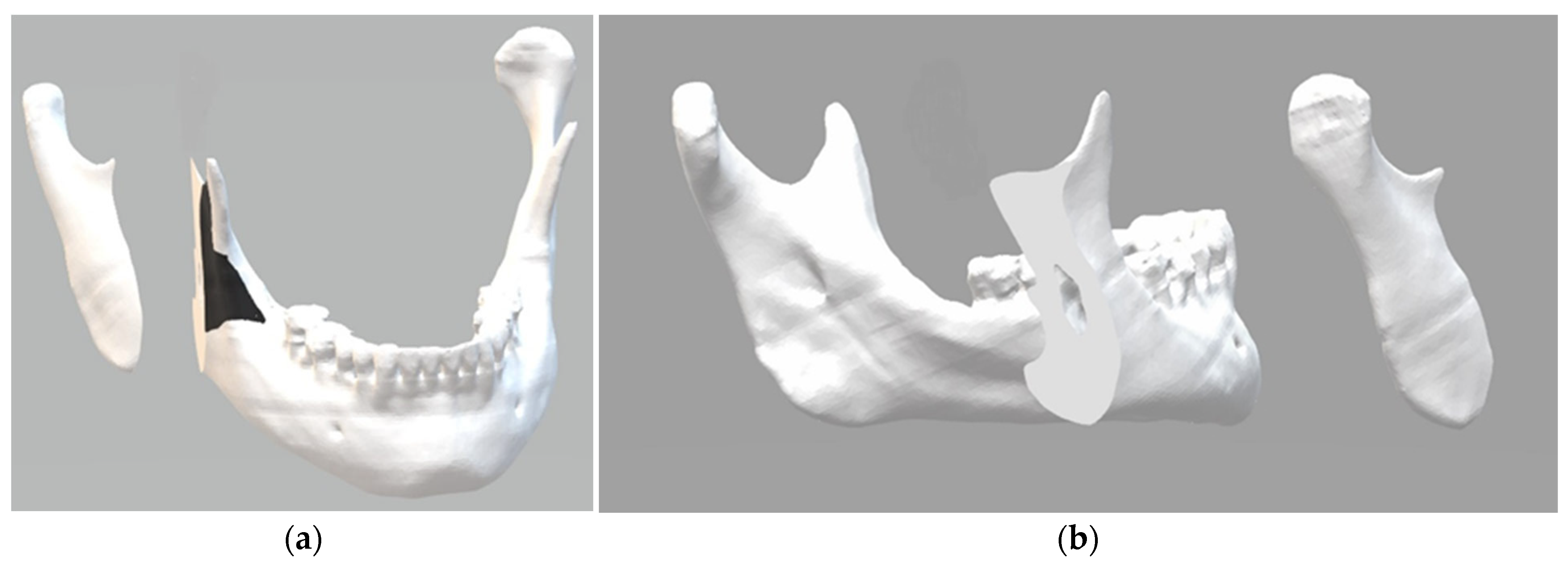
The 3D osteotomy line was determined to preserve anatomical structures and efficiently approximate and preserve surgically fractured segments in an innovative biomechanical approach. The digital mandible was sectioned according to the proposed parameters (Figure 1). The black area in the image shows where the surgical guide will be supported to perform the osteotomy. This makes it possible to determine the direction, angle, and depth of the cut to be made with the piezoelectric cutting instrument.
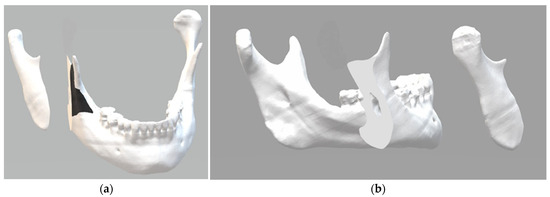
Figure 1.
Three-dimensional digital images: (a) the black area shows where the surgical guide will be supported to perform the osteotomy; (b) the surface obtained with the oblique cut of the mandibular ramus.
To validate the results, a 3D model of the mandible was printed using a material with a density like that of human bone. The described osteotomy was then performed, allowing for a comparison between the results of the digital osteotomy and the laboratory experiment, thus validating the results.
2. Materials and Methods
2.1. Materials
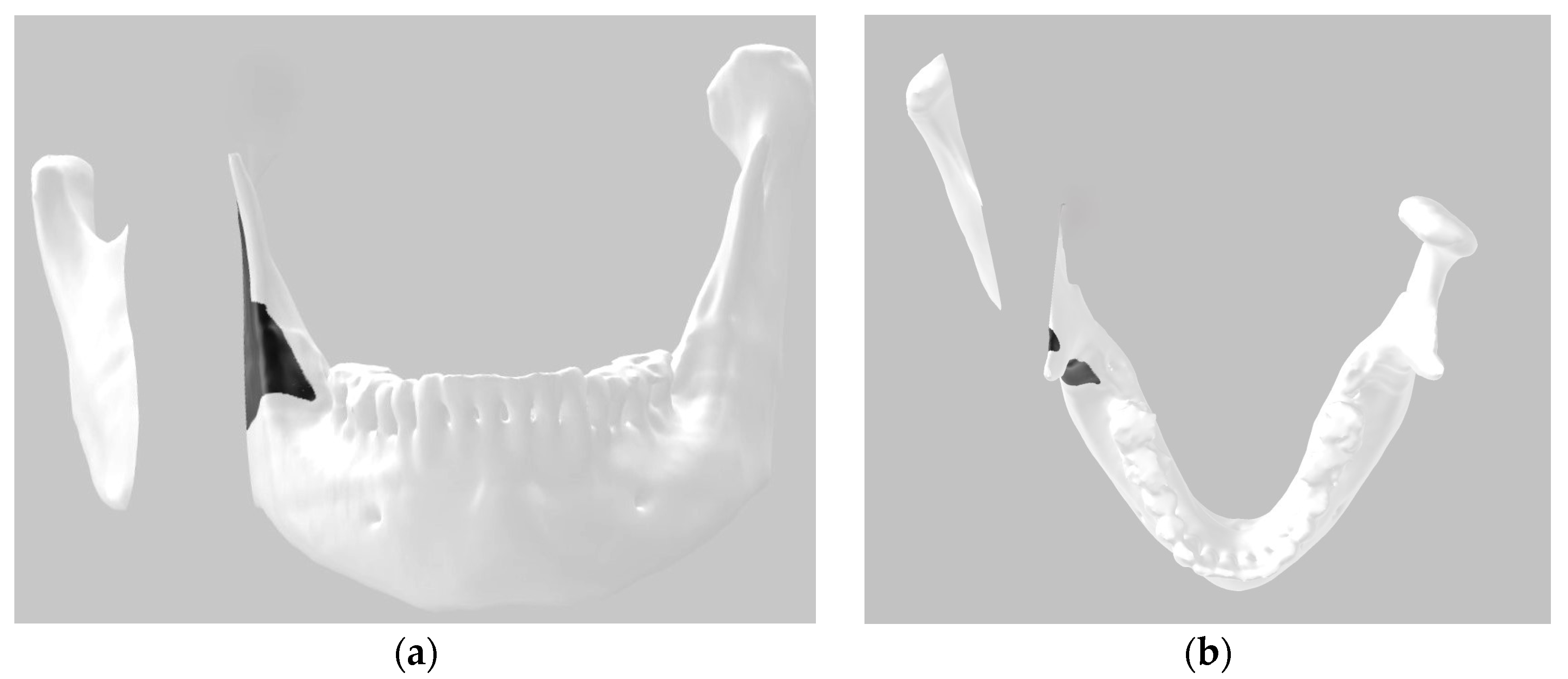
A Digital Imaging and Communications in Medicine (DICOM) image of a mandible converted into the stereolithography (STL) file format was obtained from a patient. The 3D image was segmented into three parts simulating the proposed bilateral bone section in the mandible using software available in Windows, 3D Builder (Windows 10 version), making it easy for users of this operating system to reproduce the method. The position of the surgical guide was delineated using 3D paint, which is also available in the same software (Figure 2).
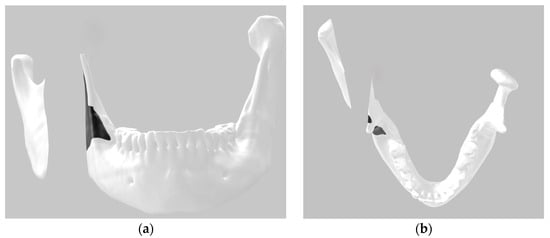
Figure 2.
Three-dimensional digital image: (a) frontal view; (b) top view.
Digital bone cutting was performed using the 3D Builder software tool to cut the digital models for 3D printing. It is possible to make three-dimensional cuts to visualize the anatomical structures to be preserved. Through trial and error, a visual analysis of the mandibular canal was made with each digital cut at different angles. On average, 50 trial cuts were needed to find the ideal osteotomy.
A model was printed using additive manufacturing in nylon (0.8 g/cm3) with a tensile modulus of 1.7 GPa, simulating the density of mandibular bone. The model was used to validate the osteotomy by cutting the bone using the author’s proposed handcrafted polymethylmethacrylate guide.
The model was subjected to cuts using a surgical motor (Tech Drill) and a 2 mm carbide cylindrical drill.
2.2. Methods
To determine the ideal bilateral mandibular osteotomy, the first parameter analyzed was the position of the foramen oval, where the mandibular nerve bundle enters the medial side or lingula of the mandibular ramus/angle. Using the posterior wall of the foramen oval as the boundary for the bone section, after marking the osteotomy line with the 3D Builder software, a digital bone section was made where it was possible to determine the preservation of the mandibular canal (Figure 3).
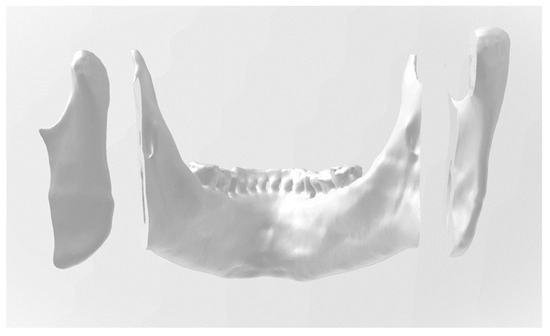
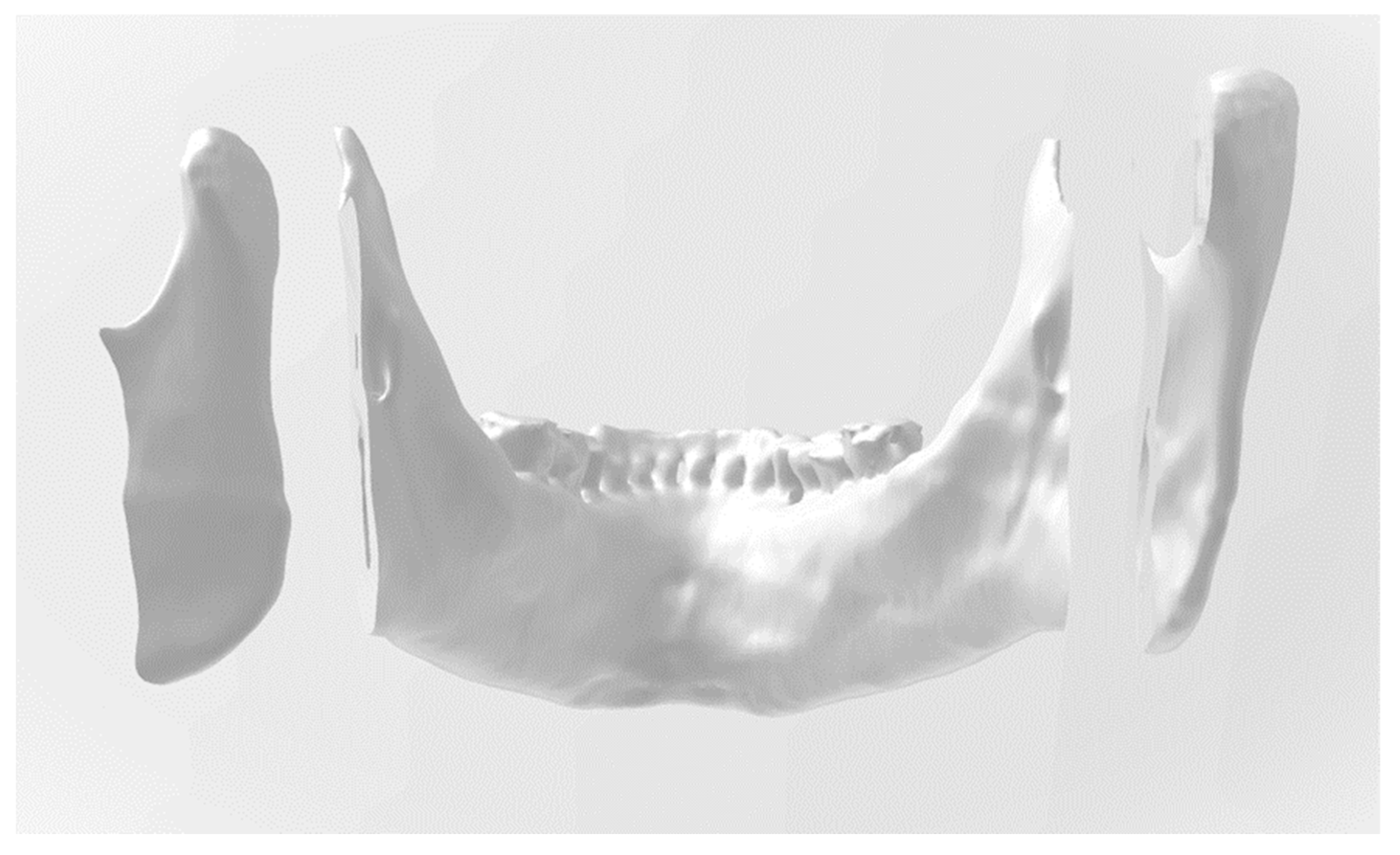
Figure 3.
Three-dimensional image that displays the mandibular dental arch.
In this figure, the 3D image displays the contact surface of the osteotomized right posterior mandibular segment, which involves the temporomandibular joint condyle. In the anterior segment, which involves the mandibular dental arch, the preservation of the mandibular canals in bilateral osteotomies is visible. The left posterior segment is visible on the right side of the picture, parallel to the incision.
The 3D mandible was printed before and after the digital cuts were made. The mandible with the bone cuts was used to make the surgical guide in vitro with methyl methacrylate (Figure 4), with the direction and angle of the cut to be made being precisely adjusted. This guide was used as a directional and angular support for the tungsten carbide drill used to perform the osteotomy. It was also possible to limit the depth of the cut, increasing the safety of the procedure in vivo.

Figure 4.
Three-dimensional mandible printed after the digital osteotomy.
The same bicortical bone cut was made on the mandible model from the glenoid fossa (Figure 5); the cut was made obliquely to facilitate the cutting angle with the surgeon’s field of view through the intraoral surgical approach and straight to the determined limit of the inferior posterior wall plus 1 mm from the foramen oval. In vivo, this bicortical cut should be made with a piezoelectric cutting instrument to preserve internal anatomical structures, especially the internal maxillary artery and the inferior alveolar vascular–nervous bundle.
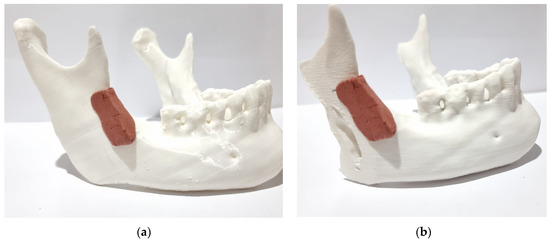
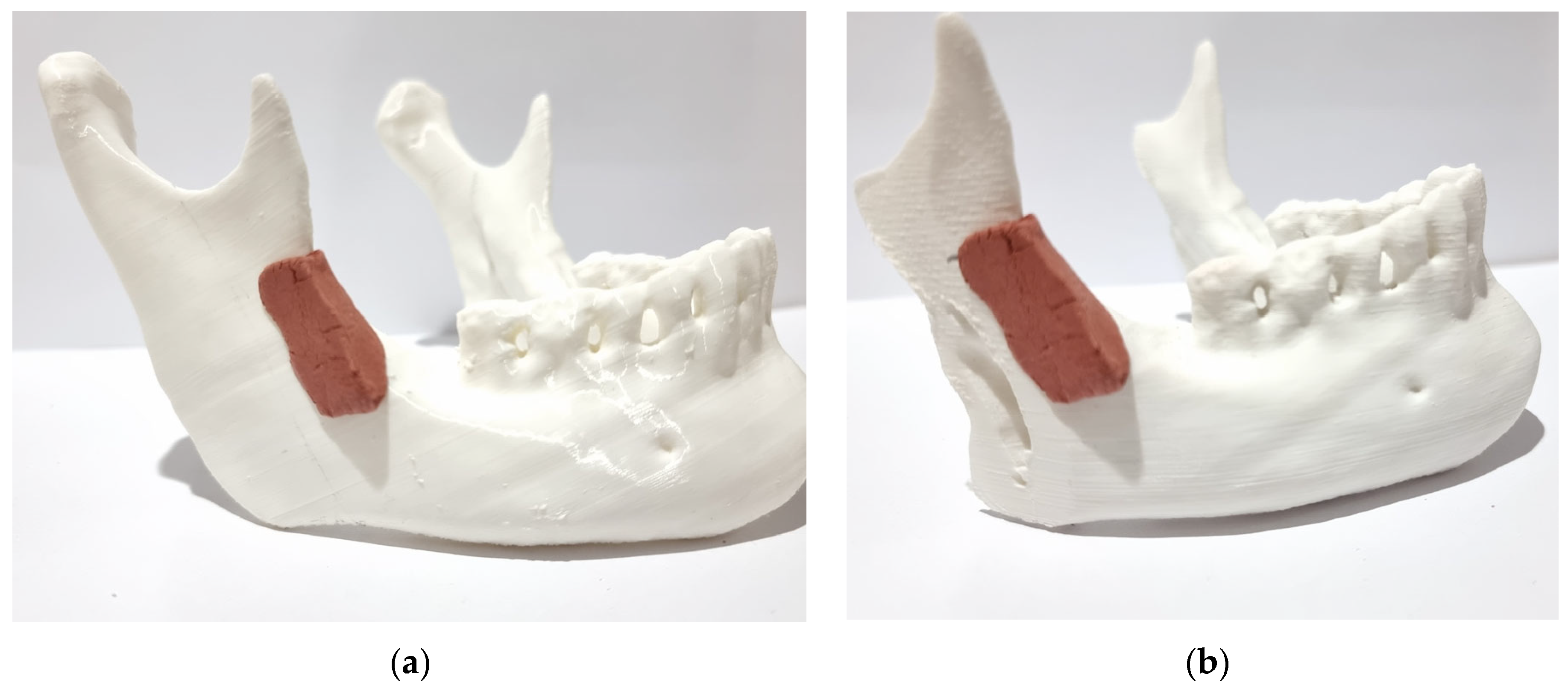
Figure 5.
Positioning of the surgical guide: (a) before osteotomy; (b) after osteotomy.
3. Results
The osteotomies made in the digital images were validated after 3D printing the models in synthetic bone (nylon) with a density of 0.8 g/cm3. The models were analyzed to ensure that the mandibular foramen and canal were preserved. The bone contact surface between the posterior and anterior segments was also validated and measured using a manual caliper with a maximum contact surface that was 20 mm wide and 57 mm high. These values provide a safety margin for mandibular retraction and advancement procedures within these limits.
The bone cut made on the synthetic bone model was accurate due to the correct angulation obtained using the surgical guide for the cut, as well as the location from the top of the mandibular notch to the final point at the edge of the mandible (Figure 5a).
The primary objective of the proposed method was to create a novel mandibular osteotomy, determined by 3D digital images and validated on a 3D printed model, which would preserve anatomical regions and, most importantly, optimize muscle action in the approximation of the osteotomized segments. This objective was successfully achieved by analyzing the final result of the osteotomized segments (Figure 5b).
The temporalis muscle will retract and elevate the anterior segment (with the mandibular dental arch), and the masseter muscle will approximate the posterior segment (mandibular ramus), maintaining contact between the osteotomized bone segments. The retention of the coronoid process (Figure 5b) in the anterior segment represents a significant biomechanical distinction when compared to the conventional techniques that are currently employed (bilateral sagittal split osteotomy).
The cut can be made with a 2 mm tungsten carbide bur, a surgical saw, or a straight piezoelectric cutting instrument. The piezoelectric device is best for the in vivo procedure, as it protects soft tissues, such as vessels and nerves.
The cutting angles applied in the osteotomy varied due to individual anatomical characteristics. This factor highlights the importance of 3D digital and laboratory technology in creating innovative guided bilateral mandibular osteotomy (GBMO).
4. Discussion
In recent years, the advantages of 3D planning and guided surgery have become apparent in maxillofacial surgery. It can be challenging for a surgeon to accurately reproduce the exact positioning of the cutting and drilling guides on the flat mandibular angles defined by the engineer, which can affect the reliability of guided bilateral sagittal split osteotomy [10].
Reference screws placed on the skeleton before the acquisition of medical computed tomographic data can serve as a fixed landmark for use during surgery and by engineers during the design phase. The results suggest that the use of reference screws is an efficient method for accurately positioning guides during guided bilateral sagittal split osteotomy [10].
Computer-aided design/computer-aided manufacturing (CAD/CAM) technologies have become increasingly popular in orthognathic surgery, especially for complex cases. These technologies are used to create cutting and drilling guides, which require virtual surgical planning and 3D modeling before they can be printed in materials such as titanium, polyamide, or resin [11].
It has been reported that a personalized titanium device can be used to support bilateral sagittal split osteotomy (BSSO) with or without genioplasty combined with individual implants for repositioning and fixation. This one-piece guide for both sides of the BSSO allows for less invasive drill placement and greater accuracy during cutting and drilling [12].
The use of this technique limited the amount of tissue detachment required and provided the necessary strength for precise bone cutting and drilling. Additionally, it facilitated the accurate fixation of preformed plates to achieve occlusion as planned virtually [11].
Since the introduction of the sagittal cut of the mandible for osteotomy of craniofacial discrepancies in 1957, few major innovations have been proposed. The only changes made have been to the angle, size, and height of the osteotomies [13].
There have been no significant innovations since that time. Suggestions for improving established techniques are mostly based on professional experience and adapting surgical procedures to individual patient needs [14].
This article presents a novel bilateral mandibular osteotomy (GBMO) that simplifies the previously performed procedure. This is accomplished using imaging technology, 3D printing, surgical guides, and bone-cutting guides.
The procedure involves a single oblique transversal cut that is guided surgically by a prefabricated model after previous surgery on models. The lingual surface of the mandibular ramus is not exposed.
The proposal is based on a digitally made cut in a 3D image, from which the surgical guide model is created. This innovative technique is used for bilateral mandibular osteotomy to advance and retract the mandible. The innovation lies in the novel design technique, rather than in the use of 3D digital models and surgical guides, which have already been extensively described in the literature.
In addition, the biomechanical advantages of the proposed technique are described by analyzing the region of the bone cut and the consequent muscular action in the posterior and anterior segments of the osteotomized mandible.
In the osteotomized posterior segment, we find the insertions of the masseter, medial pterygoid, and lateral pterygoid muscles.
The lateral pterygoid muscle is responsible for depressing the mandible, as well as assisting with protrusion and lateral movement. The depression of the mandible is largely due to gravity [15].
The medial pterygoid is a rectangular muscle with a superficial and deep head. The superficial head originates from the maxillary tuberosity of the lower jaw. The tendon fibers of the medial pterygoid insert into the angle of the mandible. The muscle assists in the elevation and protrusion of the mandible [16].
In this innovative osteotomy (GBMO), the temporalis muscle, which inserts into the mandibular coronoid process, is inserted into the osteotomized anterior segment where the mandibular dental arch is located. The anterior and mid fibers are responsible for elevating the mandible, while the posterior fibers retract it [17].
As a result, the action of the temporalis muscle lifting the mandible posteriorly helps to bring the segmented bony boundaries closer together, which is exactly the opposite of what happens with conventional bilateral sagittal cut techniques [15,16,17].
The biomechanical advantages of muscle actions in fracture stabilization, reduction, and osteotomy are well documented in the literature [8,9].
A favorable osteotomy can eliminate the need for plates and screws for bone fixation, with excellent short- and medium-term benefits for the patient and surgeon [14].
The difference between the technique proposed here and the vertical mandibular incision that was previously proposed and is still used [18,19] is the oblique transversal angle, which allows the procedure to be performed intraorally, and the fact that the angle of the incision is not vertical but defined according to the patient’s anatomy after all of the planning with digital and 3D-printed models (Figure 1).
The access to the intraoral surgical field for performing this innovative osteotomy is the same as that used for the IVRO technique or the non-oblique vertical osteotomy, requiring only minor modifications relative and inherent to the specific anatomy of each patient, like any regular intraoral surgery [20].
The method described here can also be used to train surgeons in maxillofacial surgery residencies to perform other conventional techniques, such as the bilateral sagittal split, the bilateral horizontal cut, the intraoral and/or extraoral vertical cut, and the extraoral inverted “L” osteotomy [14,21].
The limitations of the proposed technique include the need for prior training of the surgeon or the need for a technician in the team to analyze the 3D images and perform the digital bone section accurately according to predefined standards. It also adds another step in the laboratory to create a surgical guide, which should be done by a trained technician or the surgeon.
In addition, during the planning process, the surgical section must be tested on the 3D model to be printed to validate the digital planning, adding another step to the process.
The use of surgical guides as occlusal splints and or as bone-cutting guides in both the maxilla and mandible has already been shown in the literature to have advantages in terms of time savings and precision during surgery [22,23,24,25,26,27,28,29,30,31,32,33,34,35].
The most frequent complications of routinely performed mandibular osteotomies include injury to the vascular–nerve bundle of the inferior alveolar nerve, short- and medium-term mandibular position recurrences, temporomandibular joint pain and dysfunction, and infections [36,37].
The proposed technique (GBMO) aims to reduce surgical complications by minimizing the risk of injury inherent to the sagittal split procedure (Figure 6) [38,39]. During sagittal split osteotomy surgery, the mandible needs to be fractured around the inferior alveolar nerve, which unavoidably increases the risk of injury.
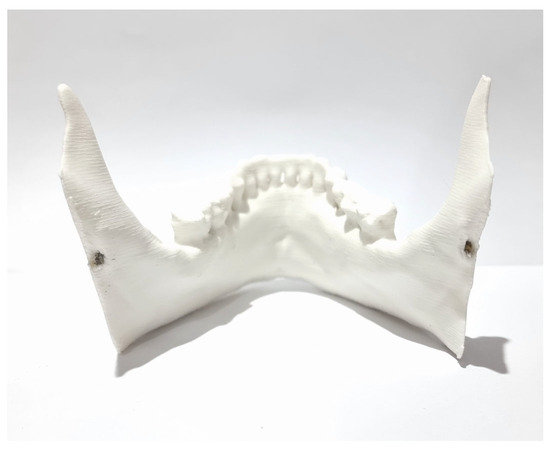
Figure 6.
Preservation of the bilateral insertion of the inferior alveolar nerve into the mandible.
In the bilateral sagittal split osteotomy, the bone cut is finalized using bone separators. However, the location and shape of the fracture in the internal region of the mandibular ramus are not standardized. This lack of standardization can result in a bad split, as mentioned in the literature [37].
The use of the finite element method and analysis of the results could provide important data and predictions, with validation of the results occurring in procedures performed in clinical trials. The implementation of the new osteotomy described here and other proposals using the same innovative method to obtain novel osteotomies based on analyses of anatomical structures and biomechanical neuromuscular forces through digital images and 3D models is the present and future of surgical technique innovation [40].
In addition to the 3D printing of monophasic and multiphase scaffolds, bioprinting and tissue engineering have emerged as innovative technologies that may change the way we view guided tissue engineering [41].
Orthognathic surgery using virtual 3D surgical planning and CAD/CAM technologies is very accurate [42]. Its application in clinical practice has increased the predictability and comfort of surgery, and it will be important for clinicians to make continuous efforts to apply the most advanced technologies that will be developed in the future to patient diagnosis and surgery [43,44,45].
The proposed method of creating a guide for the surgical bone cut was found to be safe and effective in achieving the desired outcome. The experience of the surgeon is crucial for planning and executing the digital bone section, 3D model, and future surgery.
The osteotomy procedure is proposed to be performed through an intraoral surgical approach, as extraoral approaches are not feasible with the described cutting angle.
Maintaining the insertion of the temporalis muscle in the anterior segment of the dental arch facilitates the approximation of the osteotomized anterior and posterior segments. This technique also offers the safe option of not requiring rigid fixation.
The potential challenges in clinical implementation are related to the technical training of surgeons to learn how to validate the novel osteotomy in the 3D model and to create a feasible angle for implementing the bone cut in the limited space of the mouth opening during intraoral approach surgery.
Future studies should analyze the performance of the proposed osteotomy (GBMO) by different surgeons and compare the results obtained with 3D models. A further cadaver study should then be carried out to validate the results obtained with the models, thus preparing the procedure for a clinical trial.
5. Conclusions
The utilization of digital 3D images acquired from CT scans in the form of DICOM files, which were then converted into STL files, proved to be an efficient method for developing an innovative mandibular osteotomy technique. The use of the 3D Builder software enabled the models to be digitally cut and printed before and after the digital cuts without requiring extensive computer expertise.
The described 3D osteotomy (GBMO) simplifies bilateral mandibular osteotomy by using a single and continuous oblique and transversal incision in the mandible. This preserves the fundamental anatomical regions, including the inferior alveolar nerve and the internal maxillary artery, in a safe and controlled manner.
Future studies will analyze the advantages and limitations of the technique in vivo, as well as its application in training maxillofacial surgeons in this specialty.
Author Contributions
Conceptualization, C.A.A.; methodology, C.A.A.; formal analysis, C.A.A.; investigation, C.A.A.; writing—original draft preparation, C.A.A.; writing—review and editing, E.M.M.F.; visualization, E.M.M.F.; supervision, R.N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskel Neuron Interact 2017, 17, 114–139. [Google Scholar]
- Gash, M.C.; Kandle, P.F.; Murray, I.V.; Varacallo, M. Physiology, Muscle Contraction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537140/ (accessed on 24 May 2024).
- Breeland, G.; Aktar, A.; Patel, B.C. Anatomy, Head and Neck, Mandible. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532292 (accessed on 24 May 2024).
- Basit, H.; Tariq, M.A.; Siccardi, M.A. Anatomy, Head and Neck, Mastication Muscles. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541027/ (accessed on 24 May 2024).
- Bakke, M. Mandibular elevator muscles: Physiology, action, and effect of dental occlusion. Scand. J. Dent. Res. 1993, 101, 314–331. [Google Scholar] [CrossRef]
- Klineberg, I.; Murray, G. Osseoperception: Sensory function and proprioception. Adv. Dent. Res. 1999, 13, 120–129. [Google Scholar] [CrossRef]
- Aman, J.E.; Elangovan, N.; Yeh, I.L.; Konczak, J. The effectiveness of proprioceptive training for improving motor function: A systematic review. Front. Hum. Neurosci. 2015, 8, 1075. [Google Scholar] [CrossRef]
- Panesar, K.; Susarla, S.M. Mandibular Fractures: Diagnosis and Management. Semin. Plast. Surg. 2021, 35, 238–249. [Google Scholar] [CrossRef]
- Van den Bempt, M.; Vinayahalingam, S.; Han, M.D.; Bergé, S.J.; Xi, T. The role of muscular traction in the occurrence of skeletal relapse after advancement bilateral sagittal split osteotomy (BSSO): A systematic review. Orthod. Craniofac. Res. 2022, 25, 1–13. [Google Scholar] [CrossRef]
- Philippe, B. Accuracy of position of cutting and drilling guide for sagittal split guided surgery: A proof of concept study. Br. J. Oral Maxillofac. Surg. 2020, 58, 940–946. [Google Scholar] [CrossRef]
- Savoldelli, C.; Vandersteen, C.; Dassonville, O.; Santini, J. Dental occlusal-surface-supported titanium guide to assist cutting and drilling in mandibular bilateral sagittal split osteotomy. J. Stomat. Oral Maxillofac. Surg. 2018, 119, 75–78. [Google Scholar] [CrossRef]
- Van den Bempt, M.; Liebregts, J.; Maal, T.; Bergé, S.; Xi, T. Toward a higher accuracy in orthognathic surgery by using intraoperative computer navigation, 3D surgical guides, and/or customized osteosynthesis plates: A systematic review. J. Cranio-Maxillofac. Surg. 2018, 46, 2108–2119. [Google Scholar] [CrossRef]
- Trauner, R.; Obwegeser, H. Zur Operationstechnik bei der Progenia und anderen Unterkieferanomalien. Dtsch. Zahn. Mund. Kieferhlkd. 1955, 23, 11–25. [Google Scholar]
- Andreucci, C.A. Sixty Years of Innovation in Biomechanical Orthognathic Surgery: The State of the Art and Future Directions. Osteology 2024, 4, 11–32. [Google Scholar] [CrossRef]
- Roberts, W.E.; Goodacre, C.J. The Temporomandibular Joint: A Critical Review of Life-Support Functions, Development, Articular Surfaces, Biomechanics and Degeneration. J. Prosthodont. 2020, 29, 772–779. [Google Scholar] [CrossRef]
- Li, G.W.; Liu, C.K.; Liu, P.; Deng, T.G.; Li, J.L.; Hu, K.J. Anatomical study of rat trigeminal motor nucleus-lateral pterygoid muscle projection pathway. Zhonghua Kou Qiang Yi Xue Za Zhi 2020, 55, 259–263. [Google Scholar]
- Arsenina, O.I.; Komarova, A.V.; Popova, N.V.; Popova, A.V.; Egorova, D.O. Primenenie elastokorrektora dlya ustraneniya diskoordinatsii raboty zhevatel’nykh myshts u patsientov s disfunktsiei visochno-nizhnechelyustnogo sustava [Elimination of discoordination of the masticatory muscles work in patients with muscular-articular dysfunction of the temporomandibular joint by using «elastocorrector» appliance]. Stomatologiia 2020, 99, 61–65. [Google Scholar] [CrossRef]
- Obwegeser, H.L. Orthognathic surgery and a tale of how three procedures came to be: A letter to the next generations of surgeons. Clin. Plast. Surg. 2007, 34, 331–355. [Google Scholar] [CrossRef]
- Caldwell, J.B.; Letterman, G.S. Vertical osteotomy in the mandibular rami for correction of prognathism. J. Oral. Surg. 1954, 12, 185–202. [Google Scholar]
- Peleg, O.; Mahmoud, R.; Shuster, A.; Arbel, S.; Kleinman, S.; Mijiritsky, E.; Ianculovici, C. Vertical Ramus Osteotomy, Is It Still a Valid Tool in Orthognathic Surgery? Int. J. Environ. Res. Public Health 2022, 19, 10171. [Google Scholar] [CrossRef]
- Aziz, S.R.; Greenberg, A.M.; Escobar, V.; Schwimmer, A. Mandibular Osteotomies. In Craniomaxillofacial Reconstructive and Corrective Bone Surgery; Greenberg, A., Schmelzeisen, R., Eds.; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Chakravarthy, C.; Sunder, S.; Malyala, S.K.; Tahmeen, A. 3D Printed Surgical Guides in Orthognathic Surgery—A Pathway to Positive Surgical Outcomes. In Proceedings of the International Conference on ISMAC in Computational Vision and Bio-Engineering 2018 (ISMAC-CVB), ISMAC 2018, Pandian, India, 16–17 May 2018; Pandian, D., Fernando, X., Baig, Z., Shi, F., Eds.; Lecture Notes in Computational Vision and Biomechanics; Springer: Cham, Switzerland, 2019; Volume 30. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, X.; Wang, L.; Zhang, Y.; Chen, K.; Wu, G. The Application of 3D Printing Technology for Simultaneous Orthognathic Surgery and Mandibular Contour Osteoplasty in the Treatment of Craniofacial Deformities. Aesth. Plast. Surg. 2017, 41, 1413–1424. [Google Scholar] [CrossRef]
- Wang, L.; Tian, D.; Sun, X.; Xiao, Y.; Chen, L.; Wu, G. The Precise Repositioning Instrument for Genioplasty and a Three-Dimensional Printing Technique for Treatment of Complex Facial Asymmetry. Aesth. Plast. Surg. 2017, 41, 919–929. [Google Scholar] [CrossRef]
- Rubio-Palau, J.; Prieto-Gundin, A.; Cazalla, A.A.; Serrano, M.B.; Fructuoso, G.G.; Ferrandis, F.P.; Baró, A.R. Three-dimensional planning in craniomaxillofacial surgery. Ann. Maxillofac. Surg. 2016, 6, 281–286. [Google Scholar] [CrossRef]
- Jong, W.C.; Namkug, K. Clinical Application of Three-Dimensional Printing Technology in Craniofacial Plastic Surgery. Arch. Plast. Surg. 2015, 42, 267–277. [Google Scholar] [CrossRef]
- Lin, H.H.; Lonic, D.; Lo, L. 3D printing in orthognathic surgery—A literature review. J. Formos. Med. Assoc. 2018, 117, 547–558. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2016, 31, 1116–1128. [Google Scholar] [CrossRef]
- Wrzosek, M.K.; Peacock, Z.S.; Laviv, A.; Goldwaser, B.R.; Ortiz, R.; Resnick, C.M.; Troulis, M.J.; Kaban, L.B. Comparison of time required for traditional versus virtual orthognathic surgery treatment planning. Int. J. Oral Maxillofac. Surg. 2016, 45, 1065–1069. [Google Scholar] [CrossRef]
- Tetsworth, K.; Block, S.; Glatt, V. Putting 3D modelling and 3D printing into practice: Virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. J. Soc. Int. Chir. Orthop. Traumatol. 2017, 3, 16. [Google Scholar] [CrossRef]
- Steinhuber, T.; Brunold, S.; Gärtner, C.; Offermanns, V.; Ulmer, H.; Ploder, O. Is Virtual Surgical Planning in Orthognathic Surgery Faster Than Conventional Planning? A Time and Workflow Analysis of an Office-Based Workflow for Single- and Double-Jaw Surgery. J. Oral Maxillofac. Surg. 2018, 76, 397–407. [Google Scholar] [CrossRef]
- Resnick, C.M.; Inverso, G.; Wrzosek, M.; Padwa, B.L.; Kaban, L.B.; Peacock, Z.S. Is There a Difference in Cost between Standard and Virtual Surgical Planning for Orthognathic Surgery? J. Oral Maxillofac. Surg. 2016, 74, 1827–1833. [Google Scholar] [CrossRef]
- Plooij, J.M.; Maal, T.J.J.; Haers, P.; Borstlap, W.A.; Kuijpers-Jagtman, A.M.; Berge, S.J. Digital three-dimensional image fusion processes for planning and evaluating orthodontics and orthognathic surgery. A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 341–352. [Google Scholar] [CrossRef]
- Franz, L.; Isola, M.; Bagatto, D.; Tuniz, F.; Robiony, M. A novel approach to skull-base and orbital osteotomies through virtual planning and navigation. Laryngoscope 2019, 129, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Brüllmann, D.; Schulze, R.K.W. Spatial resolution in CBCT machines for dental/maxillofacial applications—What do we know today? Dentomaxillofac. Radiol. 2015, 44, 20140204. [Google Scholar] [CrossRef] [PubMed]
- Movahed, R.; Ivory, J.W.; Delatour, F. Complications Associated with Maxillomandibular Advancement. In Management of Obstructive Sleep Apnea; Kim, K.B., Movahed, R., Malhotra, R.K., Stanley, J.J., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Steenen, S.A.; Becking, A.G. Bad splits in bilateral sagittal split osteotomy: Systematic review of fracture patterns. Int. J. Oral Maxillofac. Surg. 2016, 45, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, C.A.; Fonseca, E.M.M.; Jorge, R.N. 3D Printing as an Efficient Way to Prototype and Develop Dental Implants. BioMedInformatics 2022, 2, 671–679. [Google Scholar] [CrossRef]
- Fernandes, M.G.; Alves, J.L.; Fonseca, E.M.M. Diaphyseal femoral fracture: 3D biomodel and intramedullary nail created by additive manufacturing. Int. J. Mater. Eng. 2016, 7, 130–142. [Google Scholar] [CrossRef]
- Sioustis, I.-A.; Axinte, M.; Prelipceanu, M.; Martu, A.; Kappenberg-Nitescu, D.-C.; Teslaru, S.; Luchian, I.; Solomon, S.M.; Cimpoesu, N.; Martu, S. Finite Element Analysis of Mandibular Anterior Teeth with Healthy, but Reduced Periodontium. Appl. Sci. 2021, 11, 3824. [Google Scholar] [CrossRef]
- Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Youn, S.M.; Kim, C.Y.; Jeong, C.G.; Choi, J.Y. Surgical Accuracy of 3D Virtual Surgery and CAD/CAM-Assisted Orthognathic Surgery for Skeletal Class III Patients. J. Craniofac. Surg. 2023, 34, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ham, M.J.; Kim, W.R.; Kim, H.G.; Kwon, K.J.; Kim, S.G.; Park, Y.W. Investigating the accuracy of mandibulectomy and reconstructive surgery using 3D customized implants and surgical guides in a rabbit model. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 8. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Zhang, C.; Tian, M.; Jiang, T.; Zhang, L.; Yu, H.; Shi, J.; Wang, X. Intraoral Condylectomy with 3D-Printed Cutting Guide versus with Surgical Navigation: An Accuracy and Effectiveness Comparison. J. Clin. Med. 2023, 12, 3816. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Park, J.H.; Yang, S.; Kim, J.W. Effectiveness of individualized 3D titanium-printed Orthognathic osteotomy guides and custom plates. BMC Oral Health 2023, 23, 255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).