Computational Investigation of the Fluidic Properties of Triply Periodic Minimal Surface (TPMS) Structures in Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
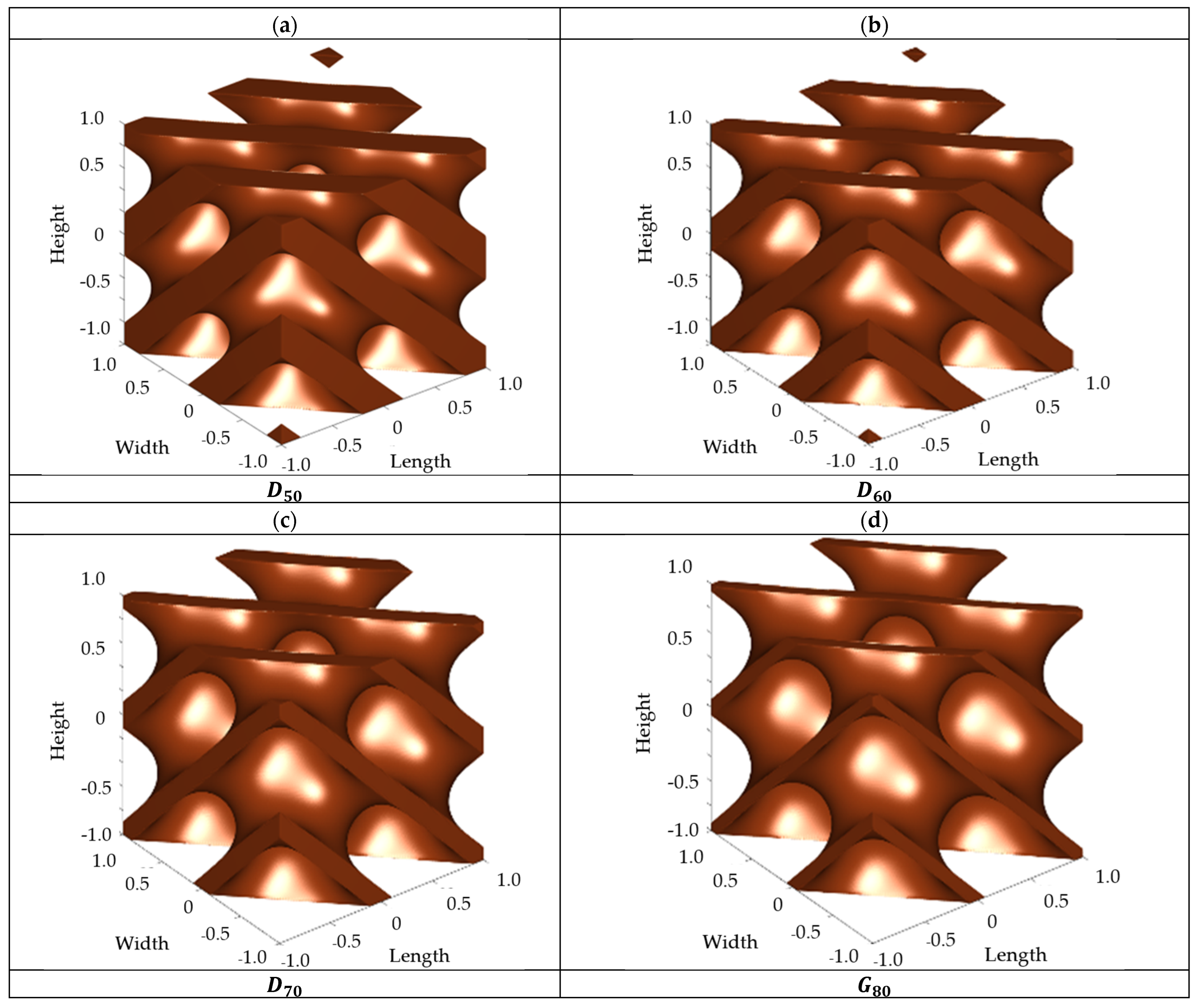
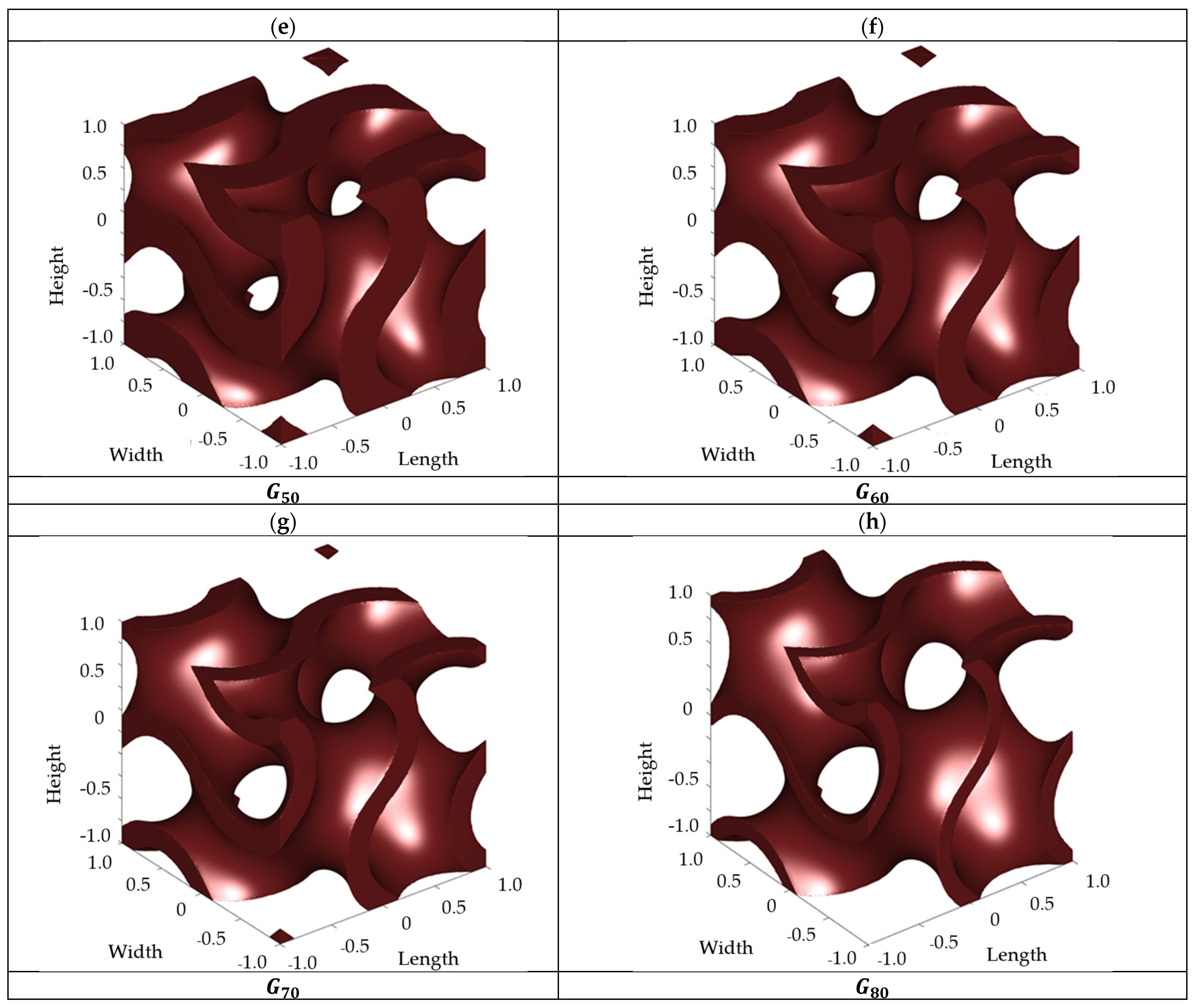
2.1. Scaffold Design
2.2. Governing and Adopted CFD Equations
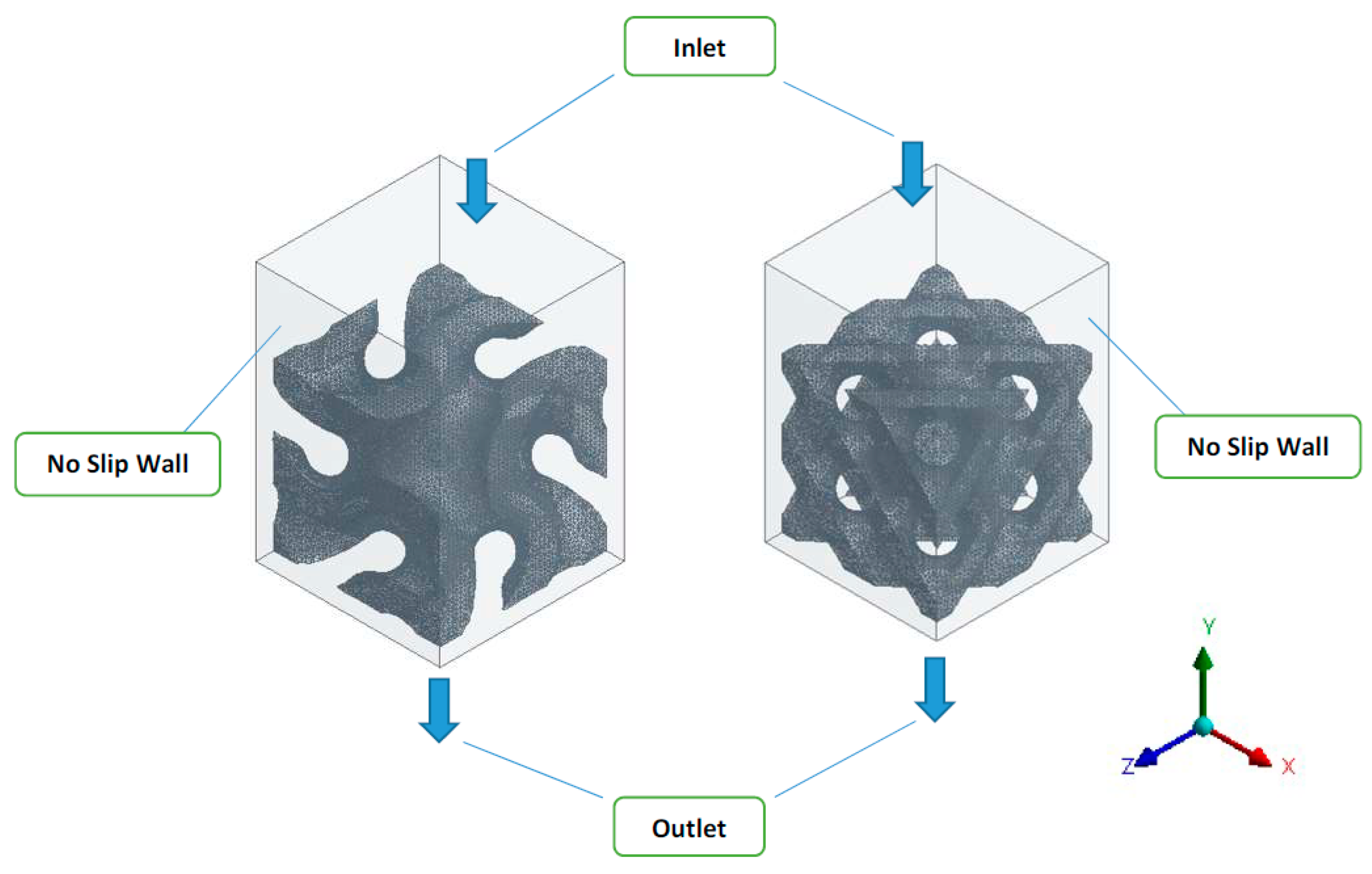
2.3. Boundary Conditions
2.4. Numerical Schemes
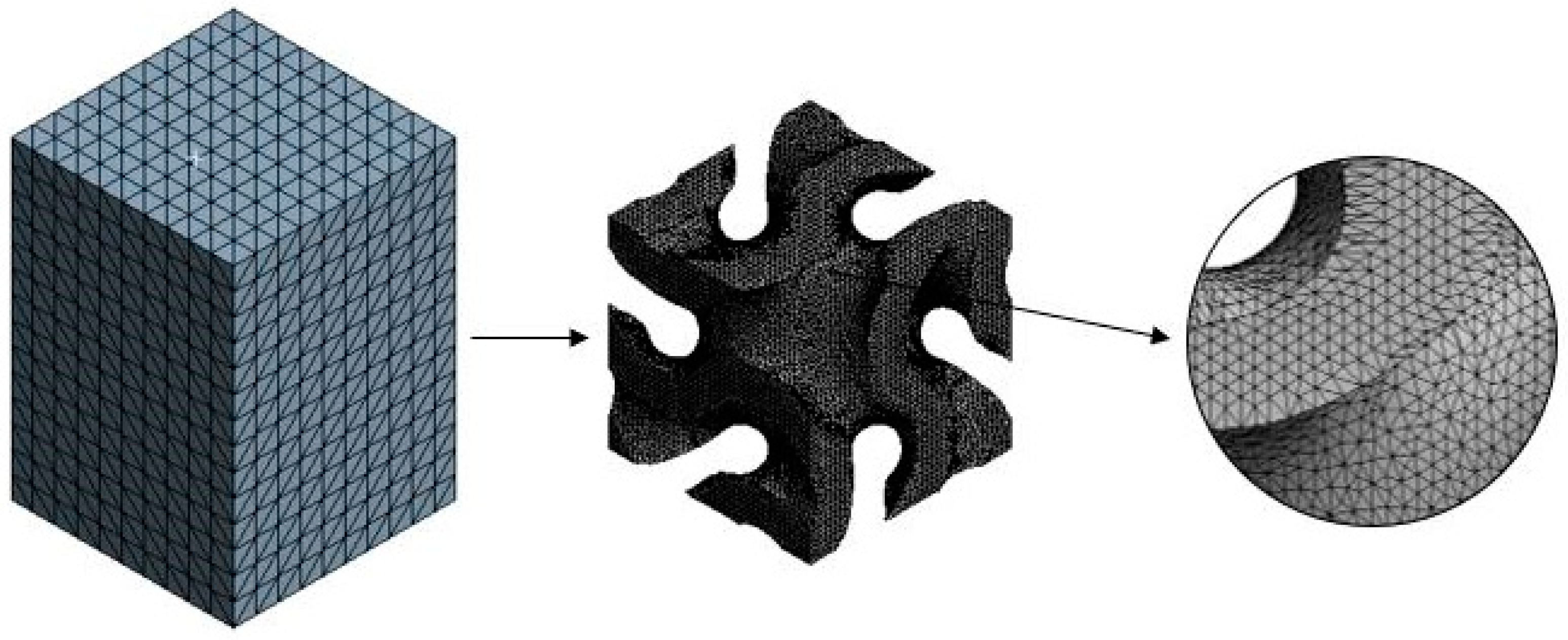
2.5. Grid Convergence
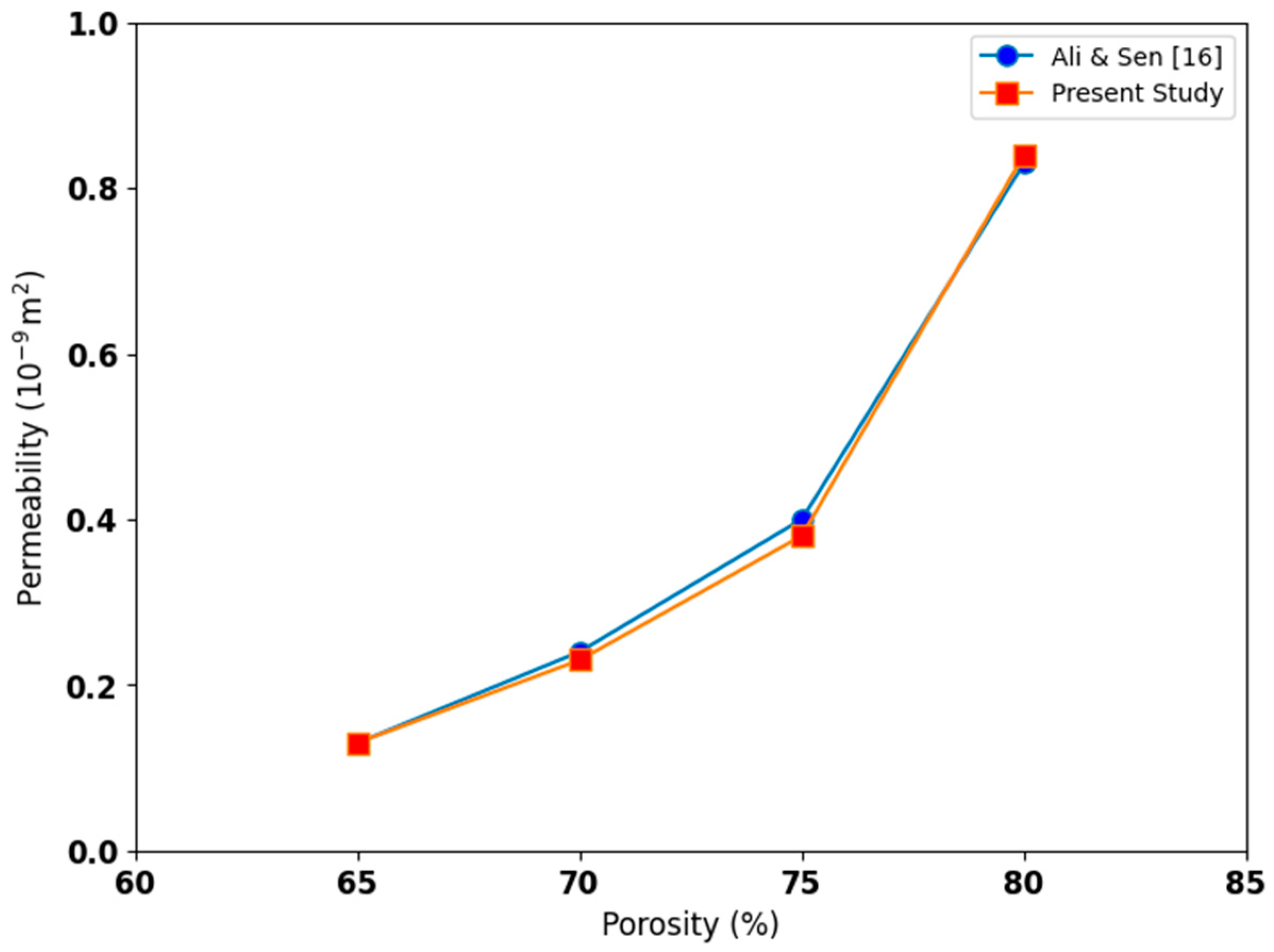
3. Numerical Model Validation
4. Results and Discussions
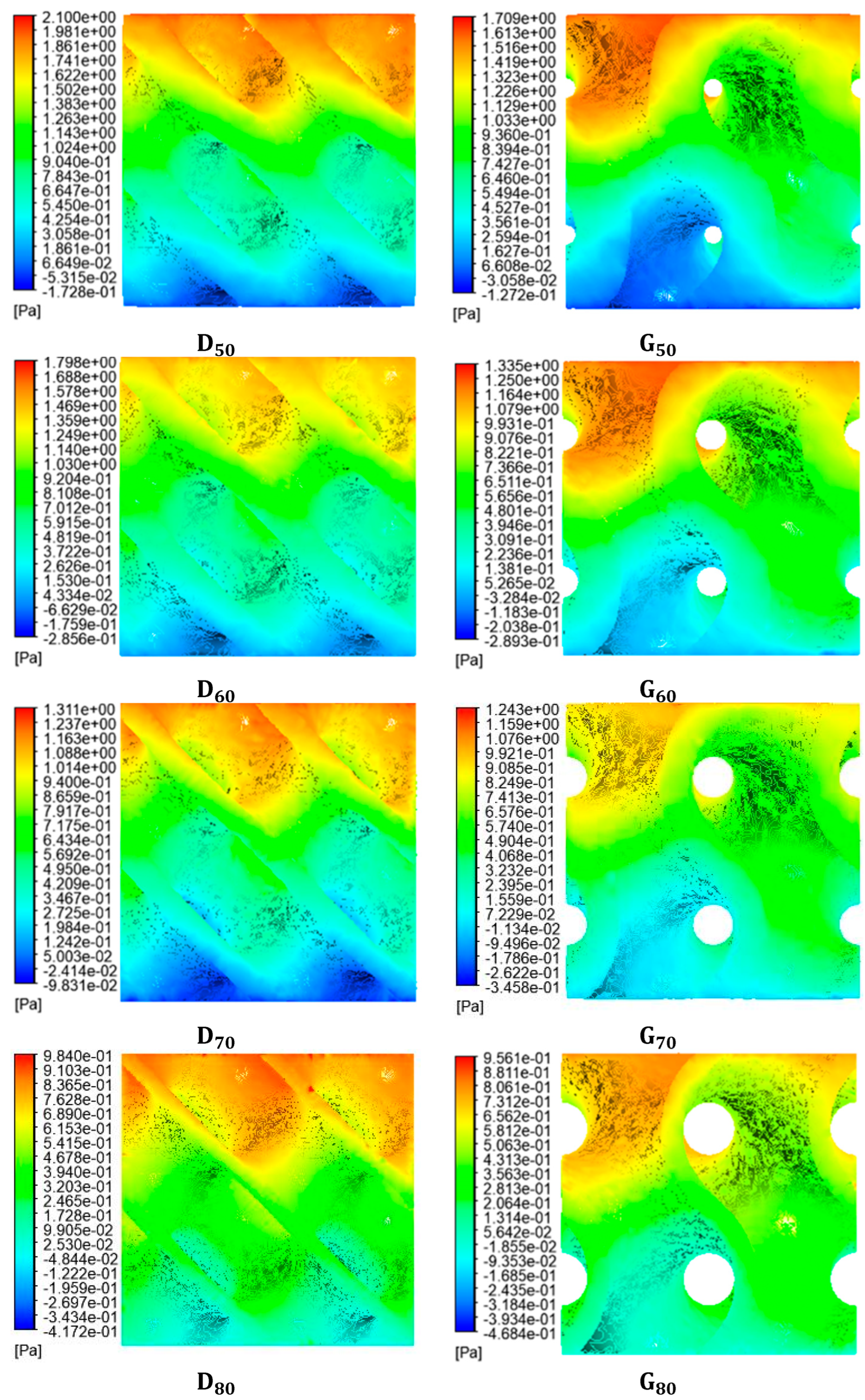
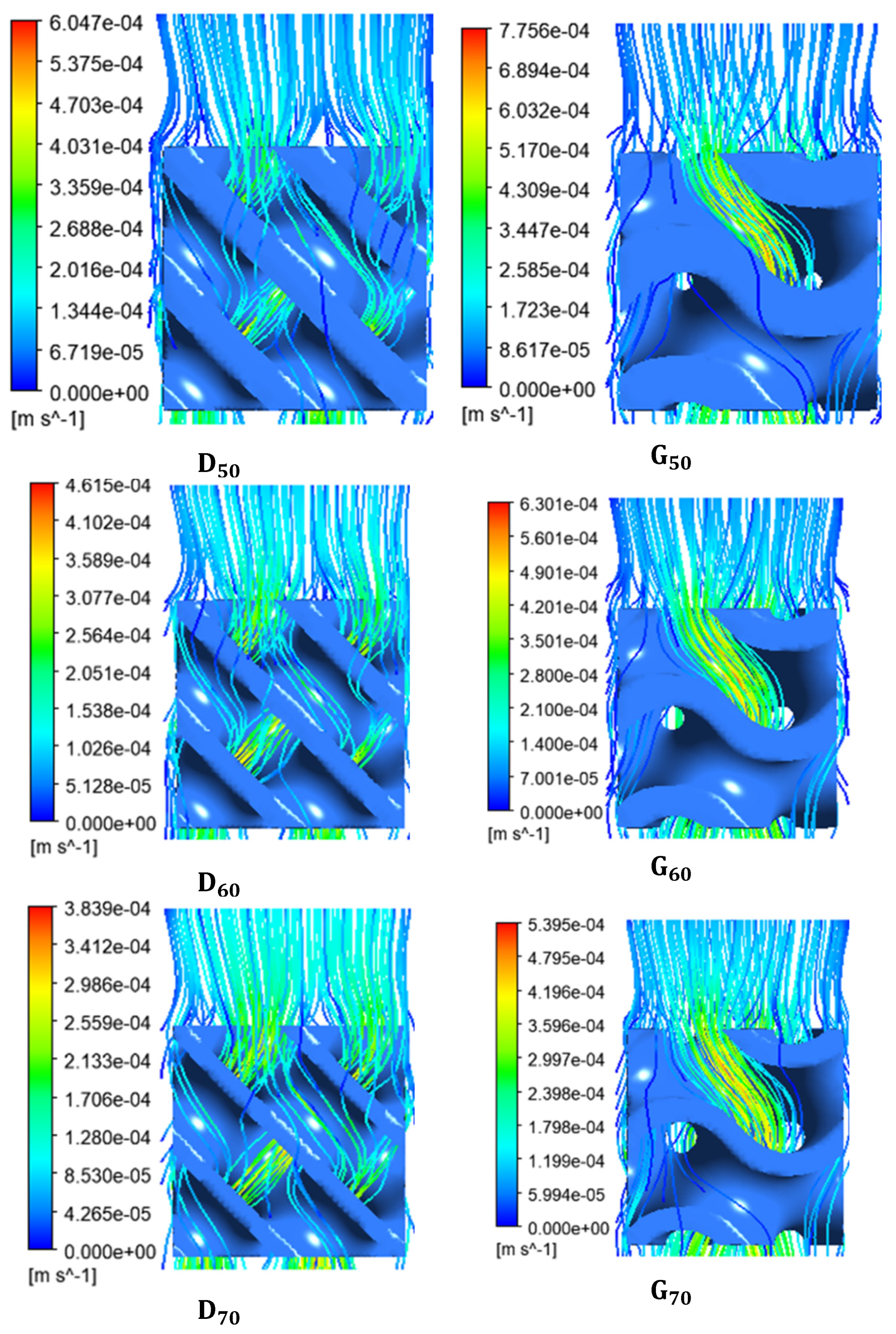
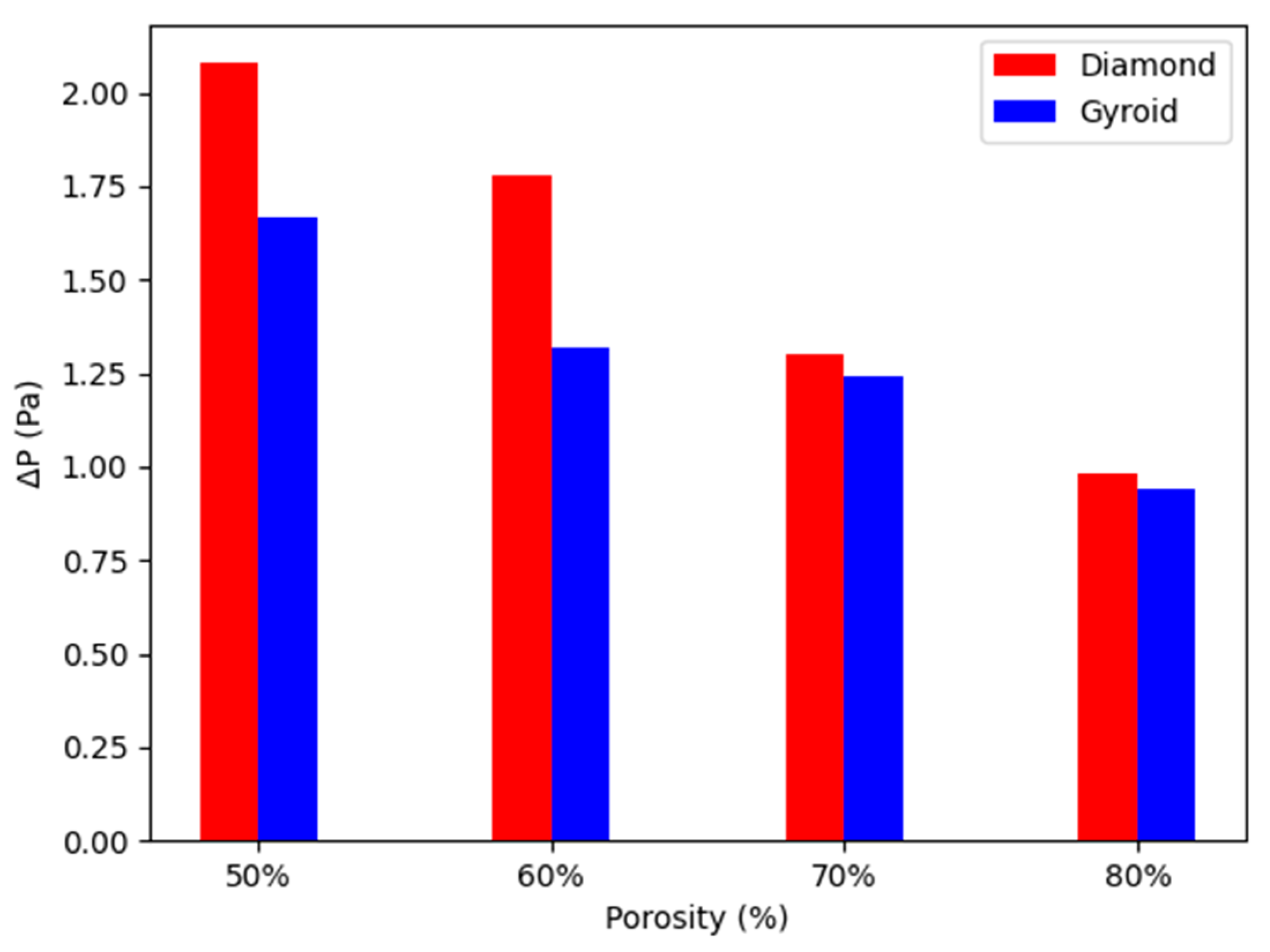
4.1. Fluidic Characteristics of Scaffolds
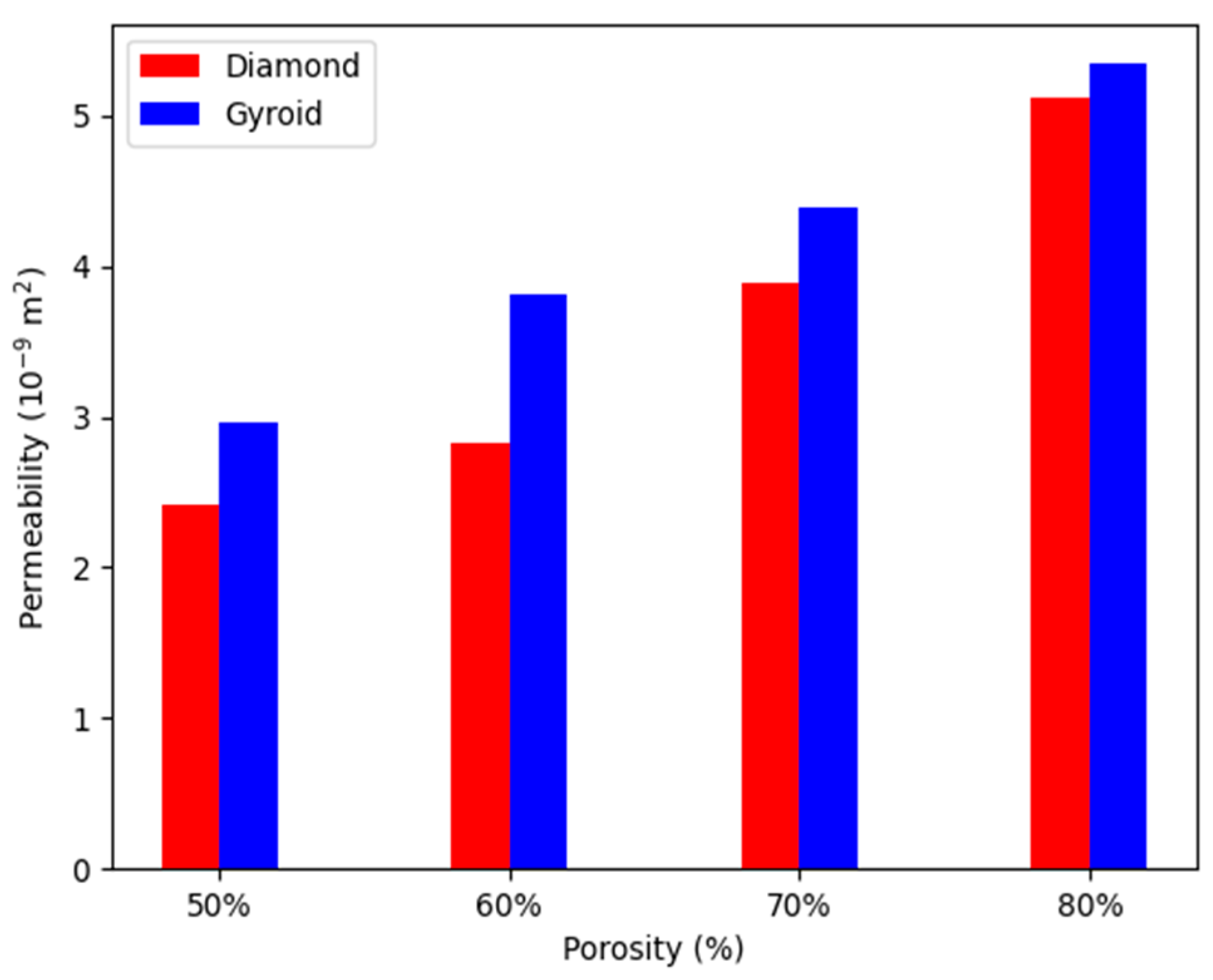
4.2. Permeability
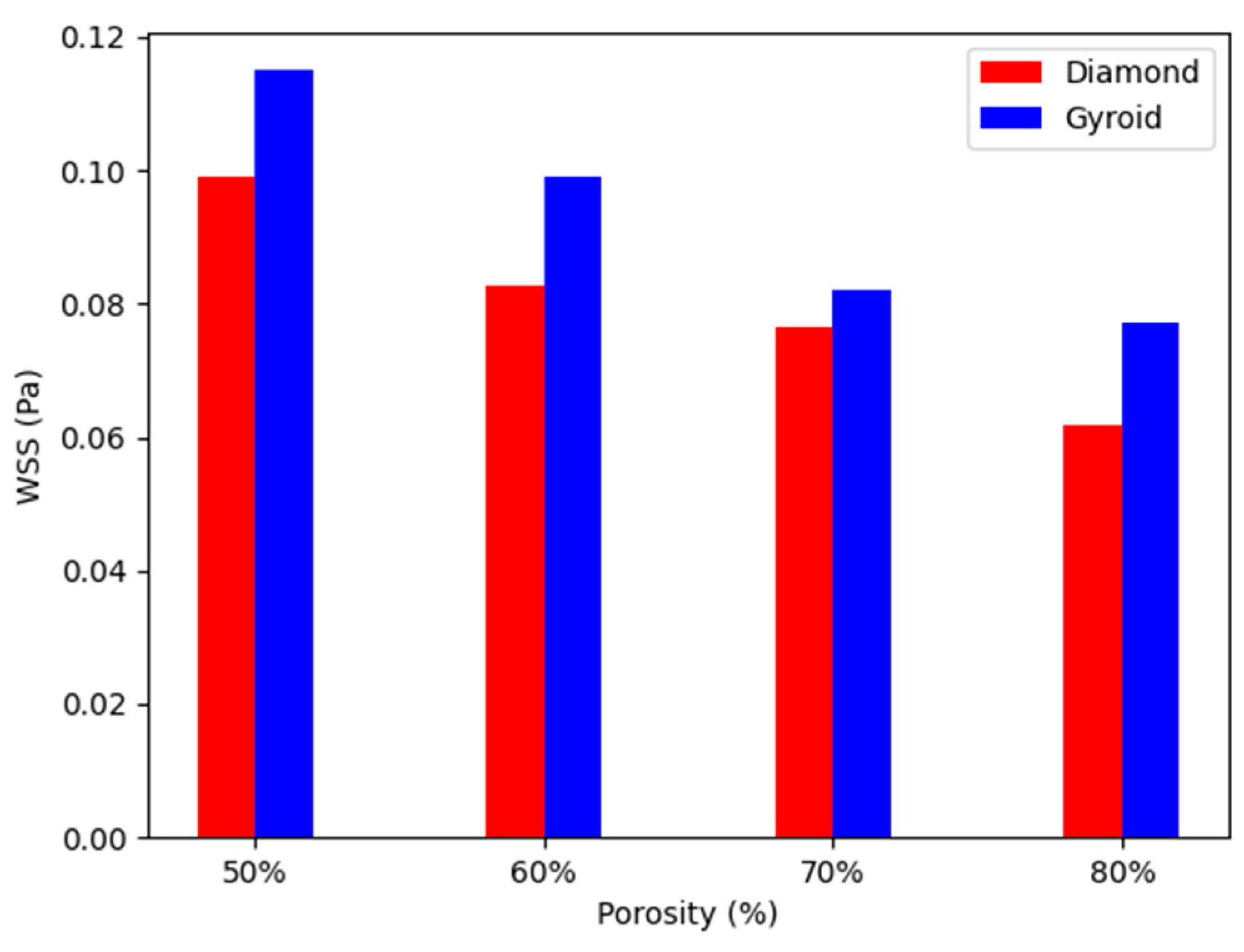
4.3. WSS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yadav, P.; Beniwal, G.; Saxena, K.K. A Review on Pore and Porosity in Tissue Engineering. Mater. Today Proc. 2021, 44, 2623–2628. [Google Scholar] [CrossRef]
- Rasheed, S.; Lughmani, W.A.; Obeidi, M.A.; Brabazon, D.; Ahad, I.U. Additive Manufacturing of Bone Scaffolds Using Polyjet and Stereolithography Techniques. Appl. Sci. 2021, 11, 7336. [Google Scholar] [CrossRef]
- Ali, D.; Sen, S. Computational Fluid Dynamics Study of the Effects of Surface Roughness on Permeability and Fluid Flow-Induced Wall Shear Stress in Scaffolds. Ann. Biomed. Eng. 2018, 46, 2023–2035. [Google Scholar] [CrossRef]
- Rasheed, S.; Lughmani, W.A.; Khan, M.M.; Brabazon, D.; Obeidi, M.A.; Ahad, I.U. The Porosity Design and Deformation Behavior Analysis of Additively Manufactured Bone Scaffolds through Finite Element Modelling and Mechanical Property Investigations. J. Funct. Biomater. 2023, 14, 496. [Google Scholar] [CrossRef]
- Castro, A.P.G.; Pires, T.; Santos, J.E.; Gouveia, B.P.; Fernandes, P.R. Permeability versus Design in TPMS Scaffolds. Materials 2019, 12, 1313. [Google Scholar] [CrossRef]
- Kumar, J.; Nirala, N.S.; Singh, N.K.; Gupta, N.; Dwivedi, Y.D.; Verma, R.; Rai, S.K.; Gupta, M. Design, Development and Fluidic Behavior Analysis of Triply Periodic Minimal Surface (TPMS) Based Scaffolds for Bone-Applications. Int. J. Interact. Des. Manuf. 2023, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, C.; Wang, J.; Wang, P.; Bi, S.; Abbas, C.A. Design and Simulation of Flow Field for Bone Tissue Engineering Scaffold Based on Triply Periodic Minimal Surface. Chin. J. Mech. Eng. 2019, 32, 19. [Google Scholar] [CrossRef]
- Poltue, T.; Karuna, C.; Khrueaduangkham, S.; Seehanam, S.; Promoppatum, P. Design Exploration of 3D-Printed Triply Periodic Minimal Surface Scaffolds for Bone Implants. Int. J. Mech. Sci. 2021, 211, 106762. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Restrepo, S.; Ocampo, S.; Ramirez, J.A.; Paucar, C.; Garcia, C. Mechanical Properties of Ceramic Structures Based on Triply Periodic Minimal Surface (TPMS) Processed by 3D Printing. J. Phys. Conf. Ser. 2017, 935, 012036. [Google Scholar] [CrossRef]
- Barba, D.; Alabort, E.; Reed, R.C. Synthetic Bone: Design by Additive Manufacturing. Acta Biomater. 2019, 97, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, A.; Montazerian, H.; Davoodi, E.; Homayoonfar, S. Predicting Permeability of Regular Tissue Engineering Scaffolds: Scaling Analysis of Pore Architecture, Scaffold Length, and Fluid Flow Rate Effects. Comput. Methods Biomech. Biomed. Engin 2017, 20, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Chabanon, M.; Duval, H.; Grenier, J.; Beauchesne, C.; Goyeau, B.; David, B. Histological Method to Study the Effect of Shear Stress on Cell Proliferation and Tissue Morphology in a Bioreactor. Tissue Eng. Regen. Med. 2019, 16, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.; Santos, J.; Ruben, R.B.; Gouveia, B.P.; Castro, A.P.G.; Fernandes, P.R. Numerical-Experimental Analysis of the Permeability-Porosity Relationship in Triply Periodic Minimal Surfaces Scaffolds. J. Biomech. 2021, 117, 110263. [Google Scholar] [CrossRef]
- Ma, S.; Tang, Q.; Han, X.; Feng, Q.; Song, J.; Setchi, R.; Liu, Y.; Liu, Y.; Goulas, A.; Engstrøm, D.S.; et al. Manufacturability, Mechanical Properties, Mass-Transport Properties and Biocompatibility of Triply Periodic Minimal Surface (TPMS) Porous Scaffolds Fabricated by Selective Laser Melting. Mater. Des. 2020, 195, 109034. [Google Scholar] [CrossRef]
- Ali, D.; Sen, S. Permeability and Fluid Flow-Induced Wall Shear Stress of Bone Tissue Scaffolds: Computational Fluid Dynamic Analysis Using Newtonian and Non-Newtonian Blood Flow Models. Comput. Biol. Med. 2018, 99, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Ozalp, M.; Blanquer, S.B.G.; Onel, S.; Permeability, S.O. Permeability and Fluid Flow-Induced Wall Shear Stress in Bone Scaffolds with TPMS and Lattice Architectures: A CFD Analysis. Eur. J. Mech. B/Fluids 2020, 79, 376–385. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Aqila, N.; Hussein, K.; Aziah, N.; Mohtar, J.; Haqim Neza, L.; Noordin, M.A.; Putra, A.; Saad, M. Pore Size Effect on Mechanical Response and Fluid Permeability for Bone Scaffold Regeneration. Mal. J. Med. Health Sci. 2021, 17, 34–39. [Google Scholar]
- Ntousi, O.; Roumpi, M.; Siogkas, P.; Deligianni, D.; Fotiadis, D.I. Computational Fluid Dynamic Analysis of Customised 3D-Printed Bone Scaffolds with Different Architectures. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; Volume 2023. [Google Scholar] [CrossRef]
- Guyot, Y.; Papantoniou, I.; Luyten, F.P.; Geris, L. Coupling Curvature-Dependent and Shear Stress-Stimulated Neotissue Growth in Dynamic Bioreactor Cultures: A 3D Computational Model of a Complete Scaffold. Biomech. Model. Mechanobiol. 2016, 15, 169–180. [Google Scholar] [CrossRef]
- Maskery, I.; Sturm, L.; Aremu, A.O.; Panesar, A.; Williams, C.B.; Tuck, C.J.; Wildman, R.D.; Ashcroft, I.A.; Hague, R.J.M. Insights into the Mechanical Properties of Several Triply Periodic Minimal Surface Lattice Structures Made by Polymer Additive Manufacturing. Polymer 2018, 152, 62–71. [Google Scholar] [CrossRef]
- Ali, D.; Sen, S. Finite Element Analysis of Mechanical Behavior, Permeability and Fluid Induced Wall Shear Stress of High Porosity Scaffolds with Gyroid and Lattice-Based Architectures. J. Mech. Behav. Biomed. Mater. 2017, 75, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Lesman, A.; Blinder, Y.; Levenberg, S. Modeling of Flow-Induced Shear Stress Applied on 3D Cellular Scaffolds: Implications for Vascular Tissue Engineering. Biotechnol. Bioeng. 2010, 105, 645–654. [Google Scholar] [CrossRef]
- Marin, A.C.; Lacroix, D. The Inter-Sample Structural Variability of Regular Tissue-Engineered Scaffolds Significantly Affects the Micromechanical Local Cell Environment. Interface Focus 2015, 5, 20140097. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Vaughan, T.J.; McNamara, L.M. Quantification of Fluid Shear Stress in Bone Tissue Engineering Scaffolds with Spherical and Cubical Pore Architectures. Biomech. Model. Mechanobiol. 2016, 15, 561–577. [Google Scholar] [CrossRef]
- Lee, H.G.; Park, J.; Yoon, S.; Lee, C.; Kim, J. Mathematical Model and Numerical Simulation for Tissue Growth on Bioscaffolds. Appl. Sci. 2019, 9, 4058. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, W.; Cui, Z.; Zhu, H.; Wu, C. The Anisotropic Elastic Behavior of the Widely-Used Triply-Periodic Minimal Surface Based Scaffolds. J. Mech. Behav. Biomed. Mater. 2019, 99, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sewify, G.H.; Javid, K.; Adeel, M.; Abbasi, A.; Khan, S.U.; Omri, M.; Kolsi, L. Blood Flow in Multi-Sinusoidal Curved Passages with Biomimetic Rheology: An Application of Blood Pumping. Mathematics 2022, 10, 1579. [Google Scholar] [CrossRef]
- Shahsavari, S.; McKinley, G.H. Mobility of Power-Law and Carreau Fluids through Fibrous Media. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2015, 92, 063012. [Google Scholar] [CrossRef]
- Cho, Y. Effects of the Non-Newtonian Viscosity of Blood on Hemodynamics of Diseased Arterial Flows. Biorheology 1991, 28, 241–262. [Google Scholar] [CrossRef]
- Truscello, S.; Kerckhofs, G.; Van Bael, S.; Pyka, G.; Schrooten, J.; Van Oosterwyck, H. Prediction of Permeability of Regular Scaffolds for Skeletal Tissue Engineering: A Combined Computational and Experimental Study. Acta Biomater. 2012, 8, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, Q.; Feng, Q.; Song, J.; Han, X.; Guo, F. Mechanical Behaviours and Mass Transport Properties of Bone-Mimicking Scaffolds Consisted of Gyroid Structures Manufactured Using Selective Laser Melting. J. Mech. Behav. Biomed. Mater. 2019, 93, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Jain, J.K.; Saxena, K.K. Design and Comprehensive Study of Biodegradable Zinc-Based Implants for Bio-Medical Applications. Adv. Mater. Process. Technol. 2022, 8, 519–536. [Google Scholar] [CrossRef]
- Naghavi, S.A.; Tamaddon, M.; Marghoub, A.; Wang, K.; Babamiri, B.B.; Hazeli, K.; Xu, W.; Lu, X.; Sun, C.; Wang, L.; et al. Mechanical Characterisation and Numerical Modelling of TPMS-Based Gyroid and Diamond Ti6Al4V Scaffolds for Bone Implants: An Integrated Approach for Translational Consideration. Bioengineering 2022, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, M.; Li, D.; Zhang, J. Deformation and Energy Absorption Performance of Functionally Graded TPMS Structures Fabricated by Selective Laser Melting. Appl. Sci. 2024, 14, 2064. [Google Scholar] [CrossRef]
- Santos, J.; Pires, T.; Gouveia, B.P.; Castro, A.P.G.; Fernandes, P.R. On the Permeability of TPMS Scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 110, 103932. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and Properties of 3D Scaffolds for Bone Tissue Engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.P.; Schrooten, J. The Effect of Pore Geometry on the in Vitro Biological Behavior of Human Periosteum-Derived Cells Seeded on Selective Laser-Melted Ti6Al4V Bone Scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Nauman, E.A.; Fong, K.E.; Keaveny, T.M. Dependence of Intertrabecular Permeability on Flow Direction and Anatomic Site. Ann. Biomed. Eng. 1999, 27, 517–524. [Google Scholar] [CrossRef]
- Beaudoin, A.J.; Mihalko, W.M.; Krause, W.R. Finite Element Modelling of Polymethylmethacrylate Flow through Cancellous Bone. J. Biomech. 1991, 24, 127–136. [Google Scholar] [CrossRef]

| TPMS Based Scaffolds | 50% | 60% | 70% | 80% |
|---|---|---|---|---|
| Diamond wall thickness (mm) | 0.42 | 0.33 | 0.25 | 0.16 |
| Gyroid wall thickness (mm) | 0.51 | 0.41 | 0.31 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, M.N.; Shahid, M.U.; Rasheed, S.; Irfan, M.; Obeidi, M.A. Computational Investigation of the Fluidic Properties of Triply Periodic Minimal Surface (TPMS) Structures in Tissue Engineering. Designs 2024, 8, 69. https://doi.org/10.3390/designs8040069
Shahid MN, Shahid MU, Rasheed S, Irfan M, Obeidi MA. Computational Investigation of the Fluidic Properties of Triply Periodic Minimal Surface (TPMS) Structures in Tissue Engineering. Designs. 2024; 8(4):69. https://doi.org/10.3390/designs8040069
Chicago/Turabian StyleShahid, Muhammad Noman, Muhammad Usman Shahid, Shummaila Rasheed, Muhammad Irfan, and Muhannad Ahmed Obeidi. 2024. "Computational Investigation of the Fluidic Properties of Triply Periodic Minimal Surface (TPMS) Structures in Tissue Engineering" Designs 8, no. 4: 69. https://doi.org/10.3390/designs8040069
APA StyleShahid, M. N., Shahid, M. U., Rasheed, S., Irfan, M., & Obeidi, M. A. (2024). Computational Investigation of the Fluidic Properties of Triply Periodic Minimal Surface (TPMS) Structures in Tissue Engineering. Designs, 8(4), 69. https://doi.org/10.3390/designs8040069







