The Adhesion and Diffusion of Saturate, Asphaltene, Resin and Aromatic (SARA) Molecules on Oxygenated and Hydrogenated Carbon Nanotubes (CNTs)
Abstract
:1. Introduction
2. Molecular Models for SARA-CNT Interface
2.1. Force Field
2.2. Molecular Model for Hydrogenated and Oxygenated CNTs
3. Simulation and Analysis Protocol for SARA-CNT System
3.1. Molecular Dynamics (MD) Protocol
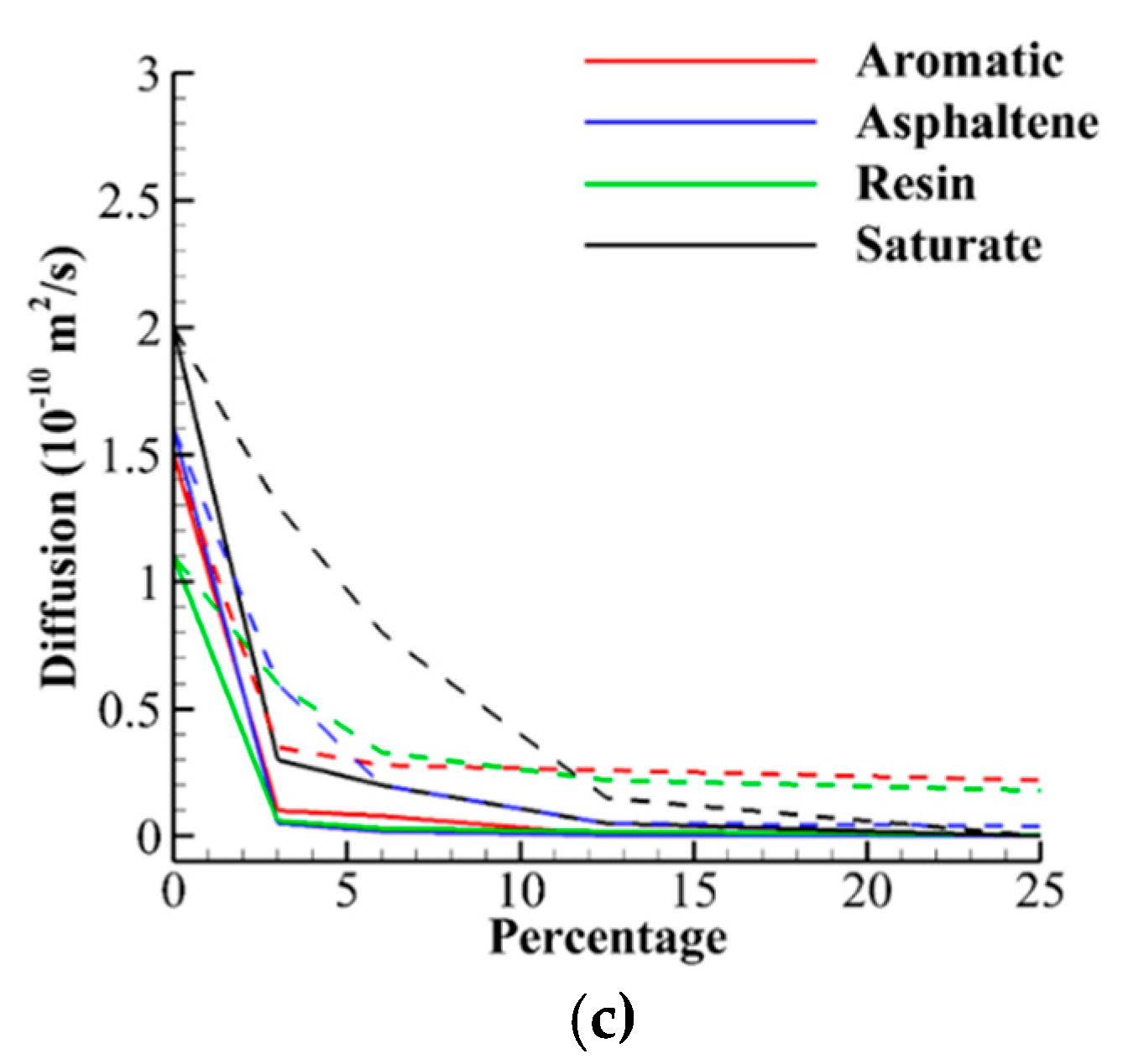
3.2. Diffusion Analysis
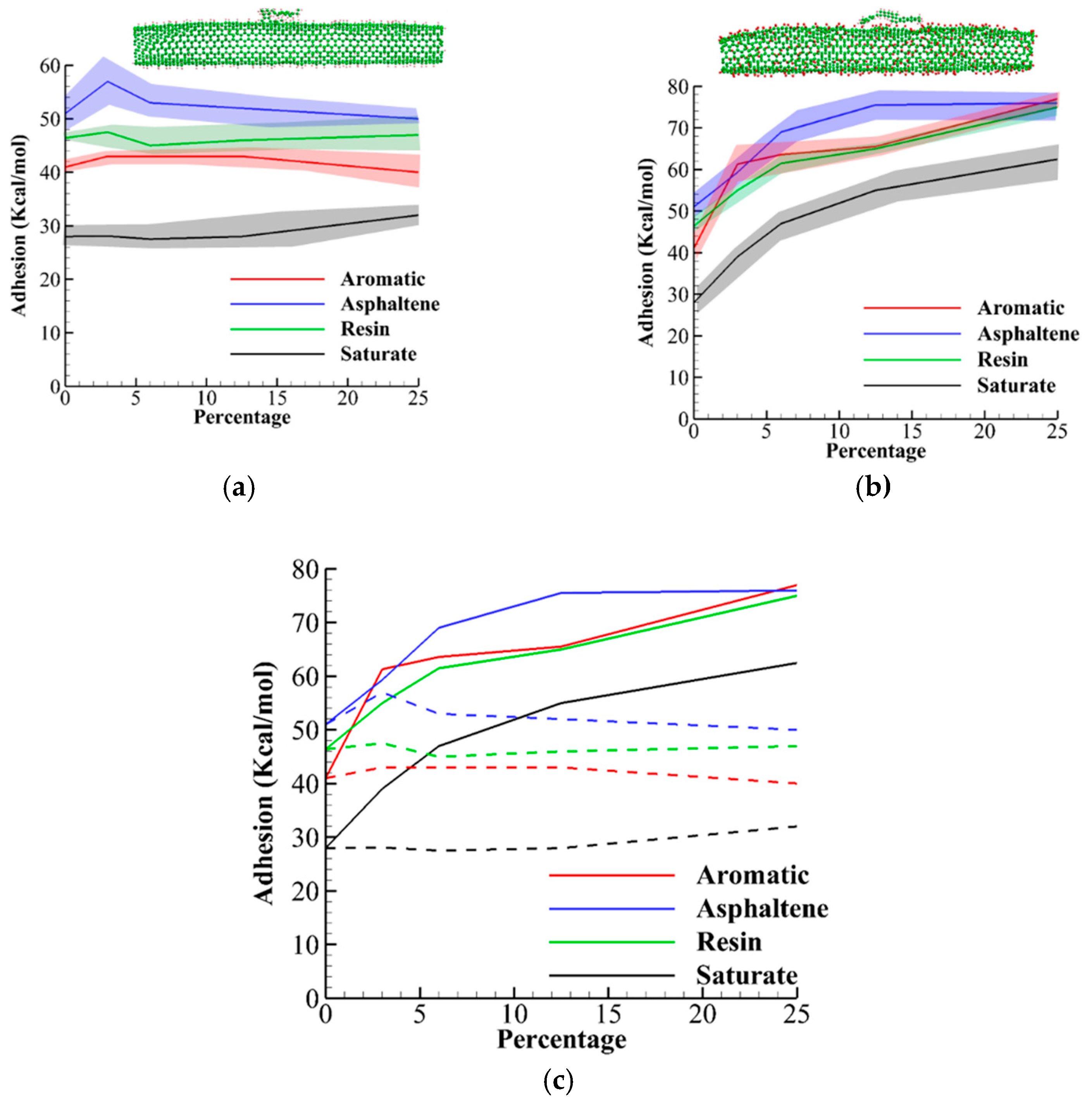
3.3. Adhesion Analysis
4. Results
5. Conclusions
- The hydrogenation has little effect on the adhesion energy, but oxygenation can increase adhesion energy significantly.
- Saturate molecule shows the highest increase in adhesion strength from 30 kcal/mol at 0% to 60 kcal/mol for 25% dosage oxygenation.
- For both hydrogenated and oxygenated CNTs at different dosage, asphaltene, resin, aromatic, and saturate molecules have the highest to lowest values, respectively.
- The diffusion coefficient of SARA molecules on both hydrogenated and oxygenated surfaces drops significantly, with lower values and more sudden drops for the latter.
- The hierarchy of diffusion coefficient values is almost the opposite of adhesion values due to larger interatomic forces; the molecules have less mobility. However, the shape of the molecule may influence the result, and more flexible molecules have more mobilities.
- The values of diffusion coefficient for asphaltene, resin, and aromatic on oxygenated CNTs with 3% dosage and higher is almost 0.0, and the molecules are stuck on the surface.
- In experiments, the surface of graphene and CNT oxides are coated with a combination of oxygen and hydroxyl (OH). Therefore this study should be seen as extreme cases where only oxygen/hydrogen exists on the surface. However, this separation reveals that oxygen has the most significant effect on adhesion and diffusion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hiremath, N.; Mays, J.; Bhat, G. Recent Developments in Carbon Fibers and Carbon Nanotube-Based Fibers: A Review. Polym. Rev. 2017, 57, 339–368. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Merum, S.; Veluru, J.B.; Seeram, R. Functionalized carbon nanotubes in bio-world: Applications, limitations and future directions. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 223, 43–63. [Google Scholar] [CrossRef]
- Shi, M.X.; Li, Q.M.; Huang, Y. Internal resonance of vibrational modes in single-walled carbon nanotubes. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 465, 3069–3082. [Google Scholar] [CrossRef] [Green Version]
- Strozzi, M.; Pellicano, F. Nonlinear Resonance Interaction between Conjugate Circumferential Flexural Modes in Single-Walled Carbon Nanotubes. Shock Vib. 2019, 2019, 3241698. [Google Scholar] [CrossRef]
- Strozzi, M.; Smirnov, V.V.; Manevitch, L.I.; Pellicano, F. Nonlinear normal modes, resonances and energy exchange in single-walled carbon nanotubes. Int. J. Non. Linear. Mech. 2020, 120, 103398. [Google Scholar] [CrossRef]
- Duch, J.; Mazur, M.; Golda-Cepa, M.; Podobiński, J.; Piskorz, W.; Kotarba, A. Insight into modification of electrodonor properties of multiwalled carbon nanotubes via oxygen plasma: Surface functionalization versus amorphization. Carbon N. Y. 2018, 137, 425–432. [Google Scholar] [CrossRef]
- Ding, Y.; Alias, H.; Wen, D.; Williams, R.A. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250. [Google Scholar] [CrossRef]
- Muthumariappan, A.; Govindasamy, M.; Chen, S.M.; Sakthivel, K.; Mani, V.; Chen, T.W.; Selvaraj, S. Determination of Non-Steroidal Anti-Inflammatory Drug (NSAID) azathioprine in human blood serum and tablet samples using Multi-Walled Carbon Nanotubes (MWCNTs) decorated manganese oxide microcubes composite film modified electrode. Int. J. Electrochem. Sci. 2017, 12, 7446–7456. [Google Scholar] [CrossRef]
- Sakthivel, K.; Govindasamy, M.; Chen, S.M.; Muthumariappan, A.; Mani, V.; Chen, T.W.; Selvaraj, S. MWCNTs/MoS2 decorated cobalt oxide polyhedrons composite film modified electrode for electrochemical determination of dopamine in rat brain and human blood serum samples. Int. J. Electrochem. Sci. 2017, 12, 7435–7445. [Google Scholar] [CrossRef]
- Mirabimoghadam, M.H.; Goli, A.; Molayem, M.; Ameri, M. Experimental study on the effect of nanosized carbon particles on fatigue resistance of asphalt binders. Pet. Sci. Technol. 2016, 34, 971–975. [Google Scholar] [CrossRef]
- Ashish, P.K.; Singh, D. Development of empirical model for predicting G∗/Sinδ and viscosity value for nanoclay and Carbon Nano Tube modified asphalt binder. Constr. Build. Mater. 2018, 165, 363–371. [Google Scholar] [CrossRef]
- Moreno-Navarro, F.; Sol-Sánchez, M.; Gámiz, F.; Rubio-Gámez, M.C. Mechanical and thermal properties of graphene modified asphalt binders. Constr. Build. Mater. 2018, 180, 265–274. [Google Scholar] [CrossRef]
- Pouranian, M.R.; Shishehbor, M. Sustainability assessment of green asphalt mixtures: A review. Environments 2019, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Qi, Z.; Li, R.; Yue, J. Investigation of the effect of aging on the thermodynamic parameters and the intrinsic healing capability of graphene oxide modified asphalt binders. Constr. Build. Mater. 2020, 230, 116984. [Google Scholar] [CrossRef]
- Ramezani, M.G.; Rickgauer, J. Understanding the adhesion properties of carbon nanotube, asphalt binder, and mineral aggregates at the nanoscale: A molecular dynamics study. Pet. Sci. Technol. 2020, 38, 28–35. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Cao, J.; Chai, J.; Cao, C.; Si, Z.; Li, Y. Adhesion between asphalt molecules and acid aggregates under extreme temperature: A ReaxFF reactive molecular dynamics study. Constr. Build. Mater. 2021, 285, 122882. [Google Scholar] [CrossRef]
- Nie, F.; Jian, W.; Lau, D. An atomistic study on the thermomechanical properties of graphene and functionalized graphene sheets modified asphalt. Carbon N. Y. 2021, 182, 615–627. [Google Scholar] [CrossRef]
- Amin, I.; El-Badawy, S.M.; Breakah, T.; Ibrahim, M.H.Z. Effect of Functionalization and Mixing Process on the Rheological Properties of Asphalt Modified with Carbon Nanotubes. Am. J. Civ. Eng. Archit. 2016, 4, 90–97. [Google Scholar]
- Huang, M.; Zhang, H.; Gao, Y.; Wang, L. Study of diffusion characteristics of asphalt–aggregate interface with molecular dynamics simulation. Int. J. Pavement Eng. 2021, 22, 319–330. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Investigation of the asphalt–aggregate interaction using molecular dynamics. Pet. Sci. Technol. 2017, 35, 586–593. [Google Scholar] [CrossRef]
- Ellul, M.D.; Gent, A.N. Role of Molecular Diffusion in the Adhesion of Elastomers. J. Polym. Sci. Part A-2 Polym. Phys. 1984, 22, 1953–1968. [Google Scholar] [CrossRef]
- Xu, W.; Qiu, X.; Xiao, S.; Hu, G.; Wang, F.; Yuan, J. Molecular dynamic investigations on the adhesion behaviors of asphalt mastic–aggregate interface. Materials 2020, 13, 5061. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Huang, W.; Xiao, Z.; Xie, J.; Hu, B.; Cai, X.; Wu, K. The Effect of Moisture on the Adhesion Energy and Nanostructure of Asphalt-Aggregate Interface System Using Molecular Dynamics Simulation. Molecules 2020, 25, 4165. [Google Scholar] [CrossRef]
- Luo, D.; Guo, M.; Tan, Y. Molecular simulation of minerals-asphalt interfacial interaction. Minerals 2018, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Wang, D.; Wang, L.; Huang, Y.; Hou, Y.; Oeser, M. The State-of-the-Art Review on Molecular Dynamics Simulation of Asphalt Binder. Adv. Civ. Eng. 2018, 2018, 4546191. [Google Scholar] [CrossRef]
- Shishehbor, M.; Pouranian, M.R.; Imaninasab, R. Evaluating the adhesion properties of crude oil fractions on mineral aggregates at different temperatures through reactive molecular dynamics. Pet. Sci. Technol. 2018, 36, 2084–2090. [Google Scholar] [CrossRef]
- Liu, J.; Yu, B.; Wang, S.; Li, L.; Zhang, J. Use of tricalcium silicate to evaluate asphalt absorption on steel slag: Atomic simulation and micro-scale characterization. Meas. J. Int. Meas. Confed. 2021, 177, 109224. [Google Scholar] [CrossRef]
- Ma, X.; Wu, J.; Liu, Q.; Ren, W.; Oeser, M. Molecular dynamics simulation of the bitumen-aggregate system and the effect of simulation details. Constr. Build. Mater. 2021, 285, 122886. [Google Scholar] [CrossRef]
- Shishehbor, M.; Pouranian, M.R.; Ramezani, M.G. Molecular investigations on the interactions of graphene, crude oil fractions and mineral aggregates at low, medium and high temperatures. Pet. Sci. Technol. 2019, 37, 804–811. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Xu, S.; Wu, S.; Liu, Q.; Fan, Z. Evaluation of thermo-mechanical properties of graphene/carbon-nanotubes modified asphalt with molecular simulation. Mol. Simul. 2017, 43, 312–319. [Google Scholar] [CrossRef]
- Ismael, M.Q.; Fattah, M.Y.; Jasim, A.F. Improving the rutting resistance of asphalt pavement modified with the carbon nanotubes additive. Ain Shams Eng. J. 2021. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Park, S.; Khalili-Araghi, F.; Tajkhorshid, E.; Schulten, K. Free energy calculation from steered molecular dynamics simulations using Jarzynski’s equality. J. Chem. Phys. 2003, 119, 3559–3566. [Google Scholar] [CrossRef]

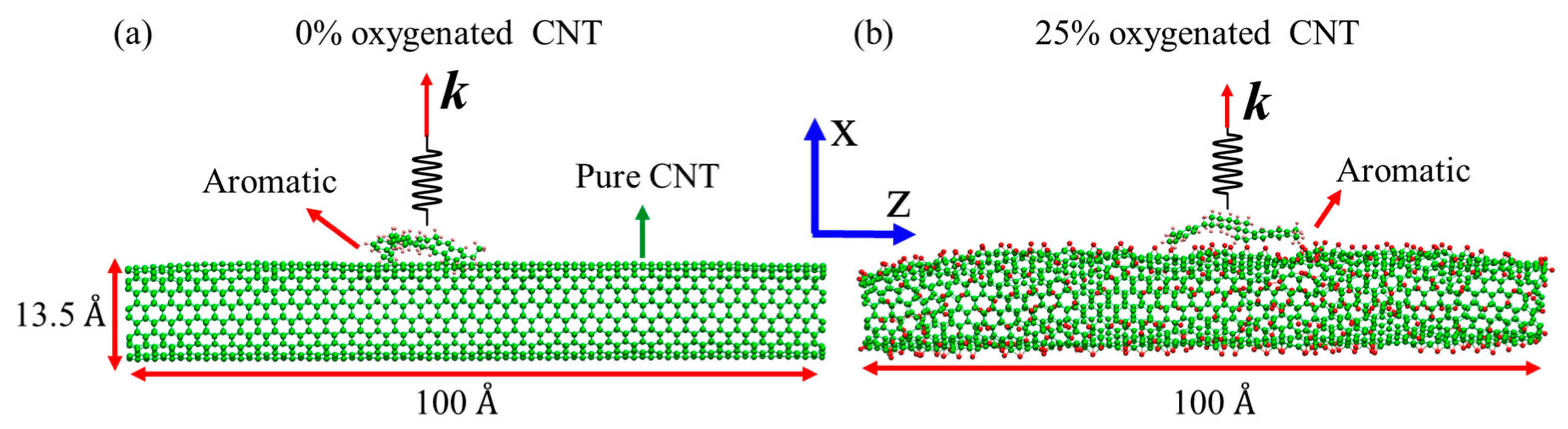

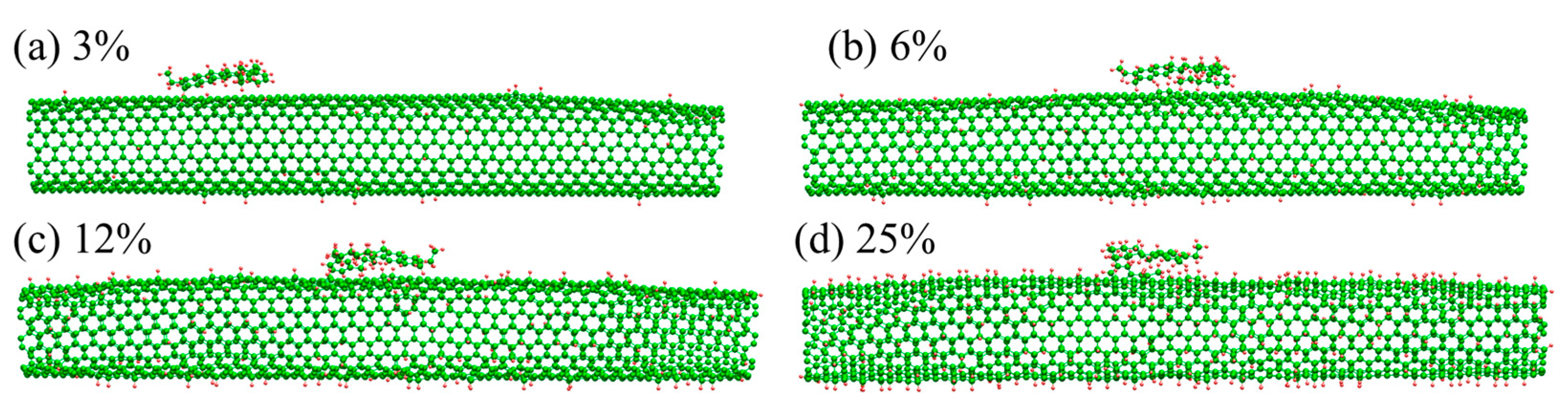


| Molecule | Number of Atoms | Molecular Weight (amu) | Molecular Area (Å2) |
|---|---|---|---|
| Saturate | 97 | 480 | 170 |
| Asphaltene | 97 | 575 | 255 |
| Resin | 100 | 555 | 200 |
| Aromatic | 79 | 465 | 205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishehbor, M.; Esmaeeli, H.S.; Pouranian, M.R. The Adhesion and Diffusion of Saturate, Asphaltene, Resin and Aromatic (SARA) Molecules on Oxygenated and Hydrogenated Carbon Nanotubes (CNTs). Infrastructures 2021, 6, 123. https://doi.org/10.3390/infrastructures6090123
Shishehbor M, Esmaeeli HS, Pouranian MR. The Adhesion and Diffusion of Saturate, Asphaltene, Resin and Aromatic (SARA) Molecules on Oxygenated and Hydrogenated Carbon Nanotubes (CNTs). Infrastructures. 2021; 6(9):123. https://doi.org/10.3390/infrastructures6090123
Chicago/Turabian StyleShishehbor, Mehdi, Hadi S. Esmaeeli, and M. Reza Pouranian. 2021. "The Adhesion and Diffusion of Saturate, Asphaltene, Resin and Aromatic (SARA) Molecules on Oxygenated and Hydrogenated Carbon Nanotubes (CNTs)" Infrastructures 6, no. 9: 123. https://doi.org/10.3390/infrastructures6090123
APA StyleShishehbor, M., Esmaeeli, H. S., & Pouranian, M. R. (2021). The Adhesion and Diffusion of Saturate, Asphaltene, Resin and Aromatic (SARA) Molecules on Oxygenated and Hydrogenated Carbon Nanotubes (CNTs). Infrastructures, 6(9), 123. https://doi.org/10.3390/infrastructures6090123





