Antibacterial Activity of Polypropylene Meshes for Hernioplasty with Ag and (Ag,Cu) Coatings Deposited via Magnetron Sputtering
Abstract
:1. Introduction
- Biocompatibility;
- Bioresistance;
- Resistance to infection;
- Mechanical strength;
- Ability to rapidly grow into tissues;
- Limited stretchability in all directions;
- Resistance to the unraveling and crumbling of edges;
- Softness and good modellability;
- Minimal material consumption;
- The retention of consumer properties after sterilization.
| Material | Ag | Al | Cu | Ta | Cr | Au | Mo | W |
|---|---|---|---|---|---|---|---|---|
| Thermal energy, eV/atom | 7–10 | 13 | 17 | 20 | 20 | 23 | 47 | 73 |
| Substrate temperature, °C | 50 | 79 | 110 | 97 | 118 | 106 | 163 | 202 |
| Deposition rate, nm/s | 44 | 13 | 30 | 8 | 17 | 37 | 12 | 8 |
2. Materials and Methods
| Sample Designation | Ag | Cu | Ar | N2 | Glow-Discharge Etching [64,65] |
|---|---|---|---|---|---|
| N1 | + | + | + | - | - |
| N2 | + | - | - | + | - |
| N3 | + | - | - | + | + |
| N4 | + | + | - | + | - |
| N5 | + | + | - | + | + |
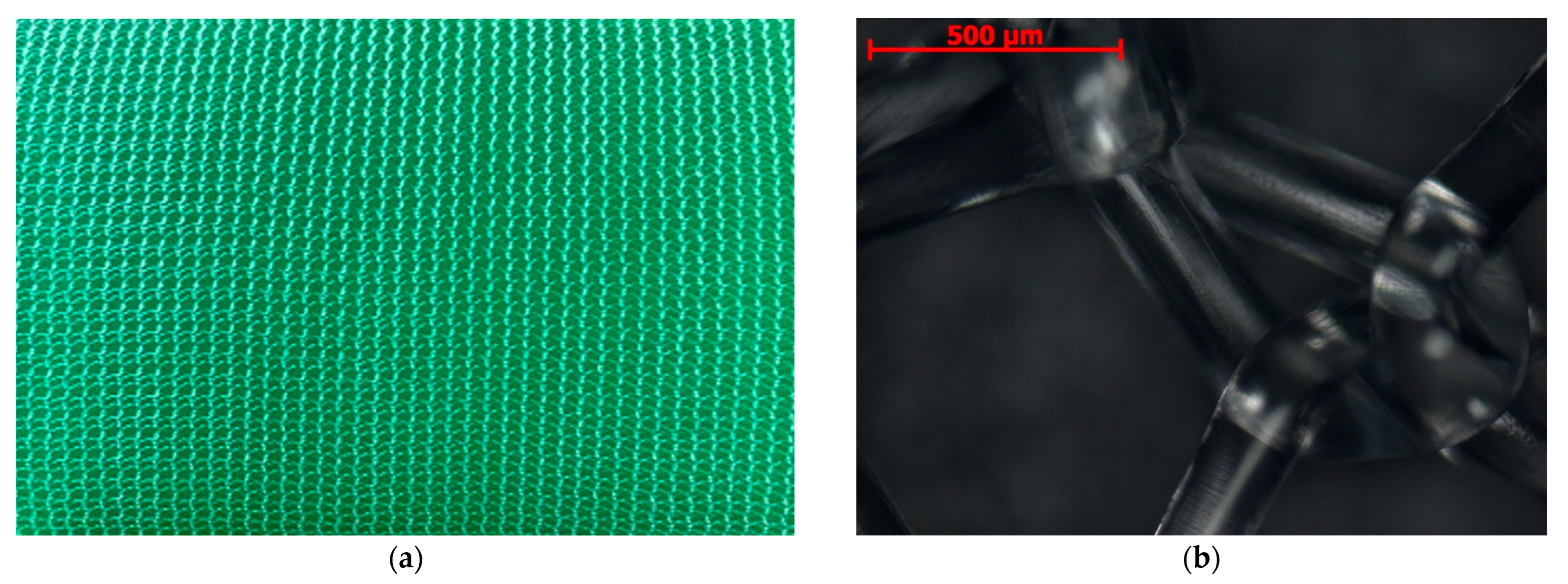
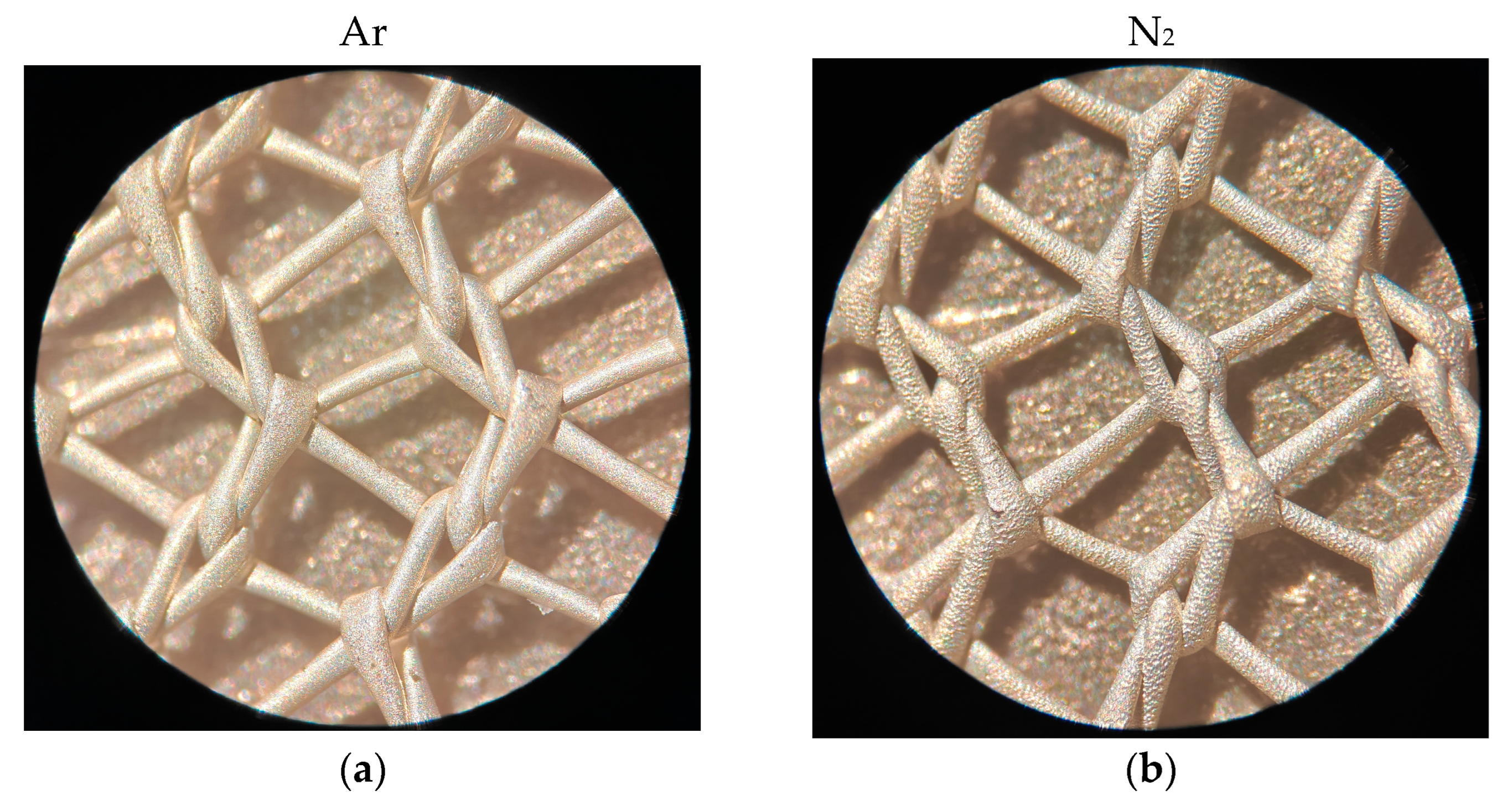
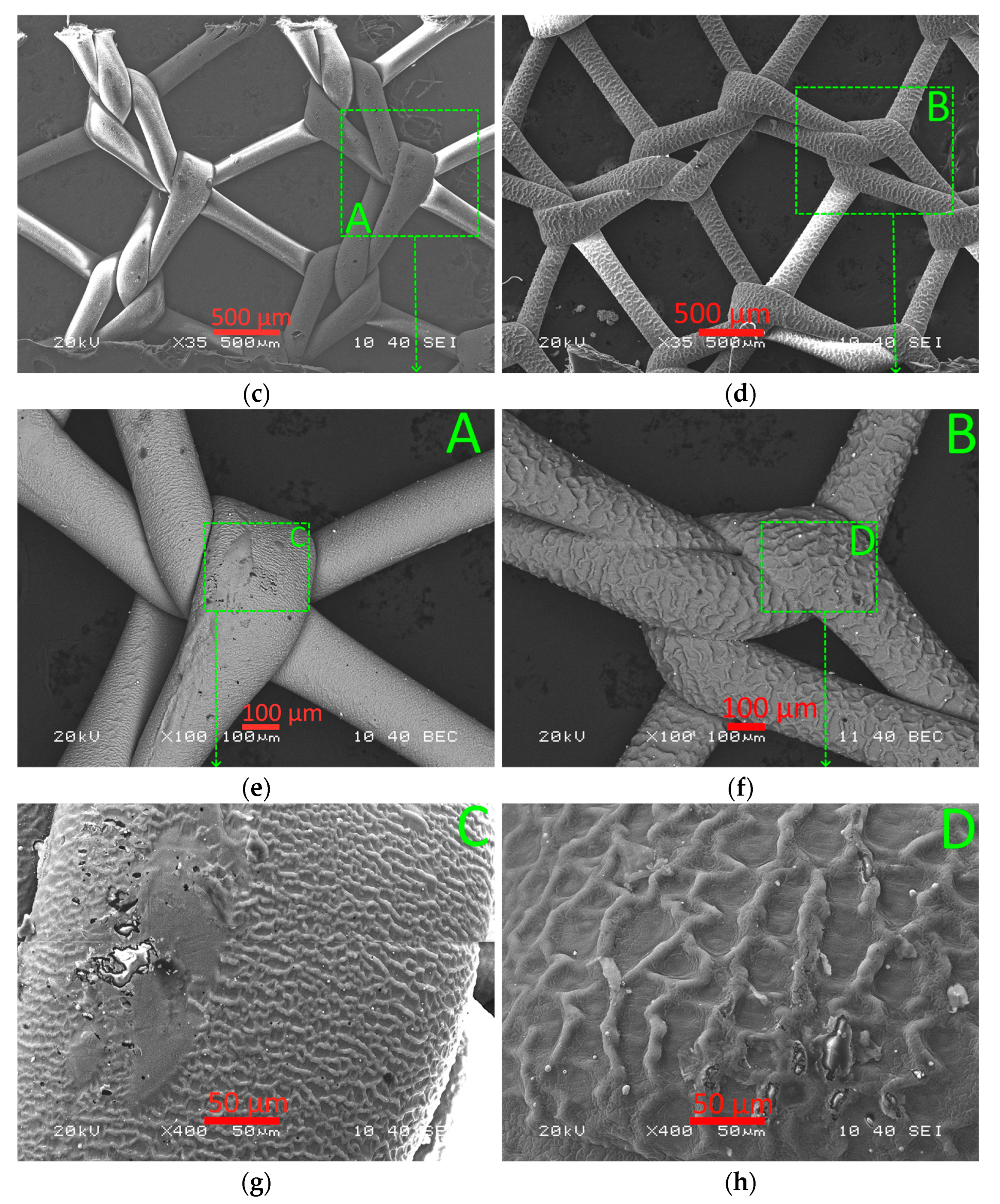
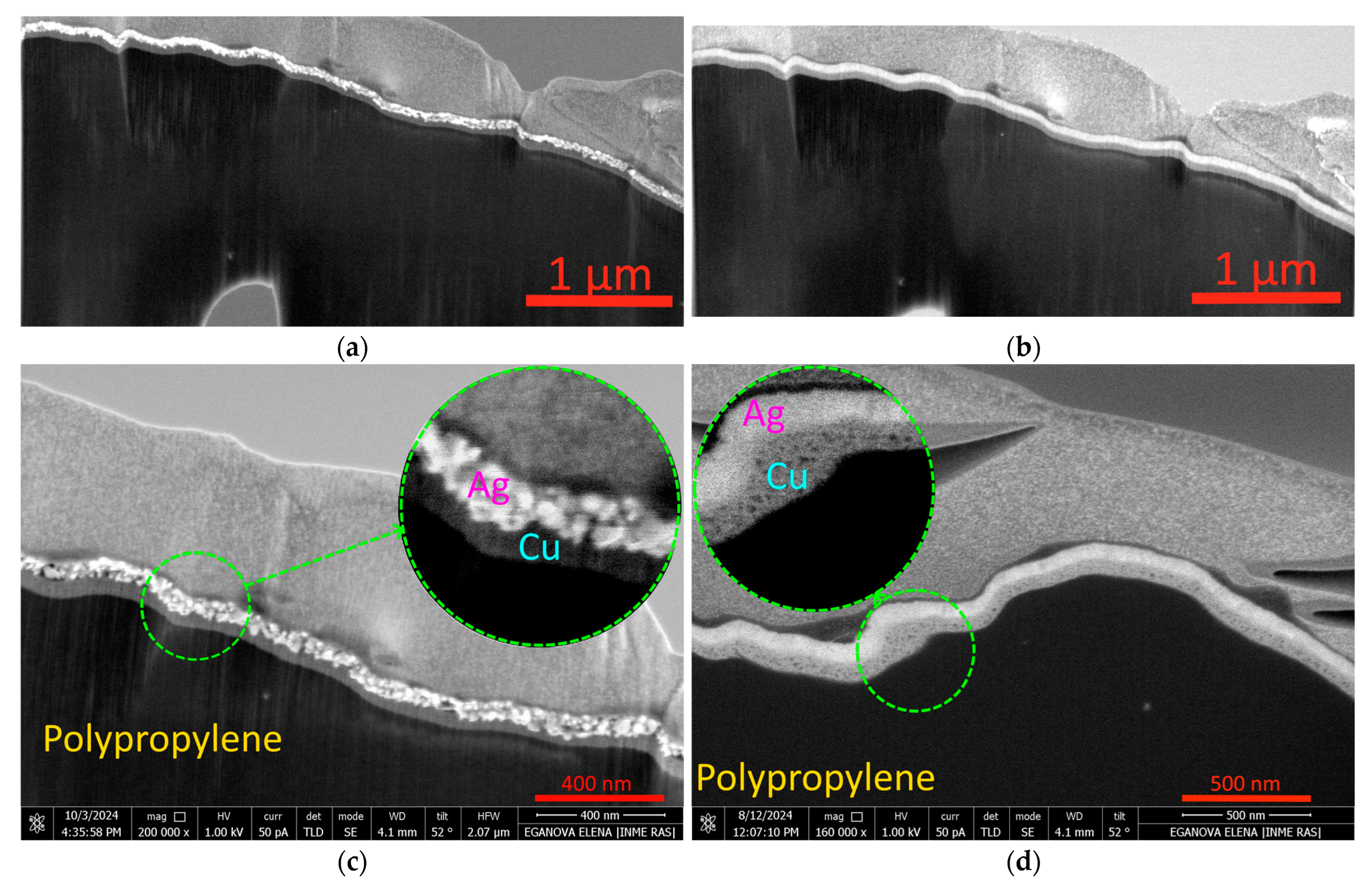
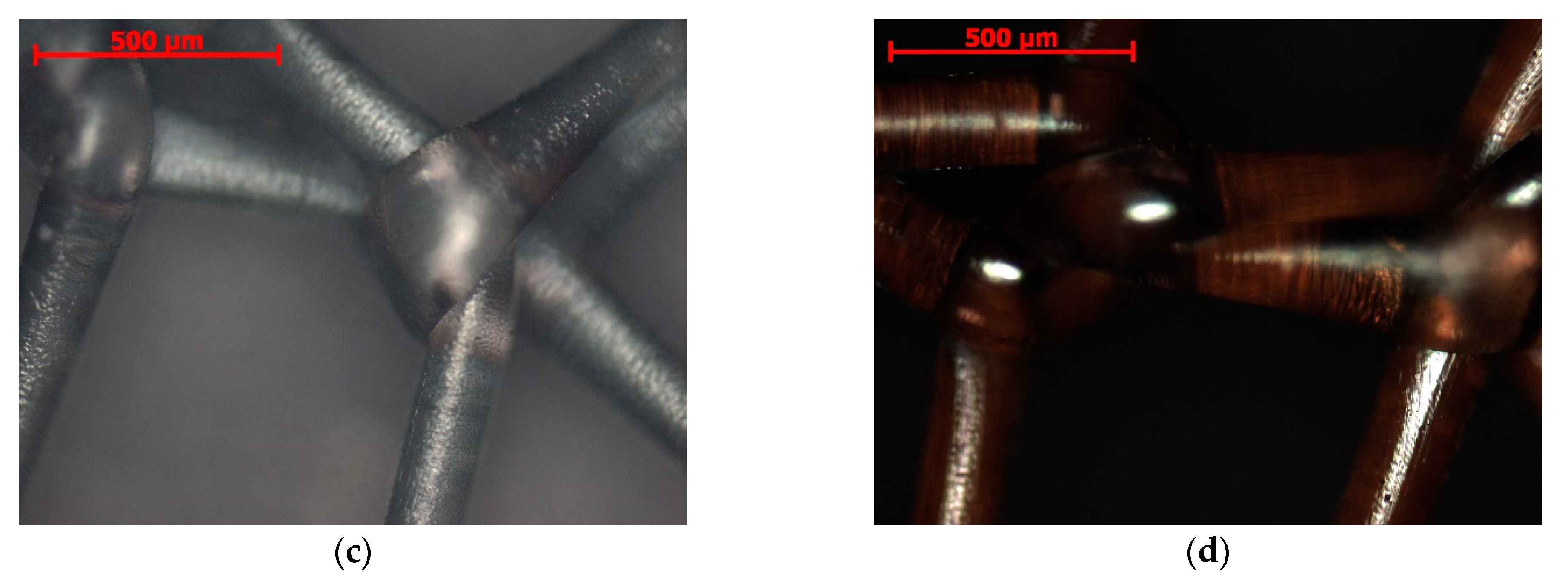
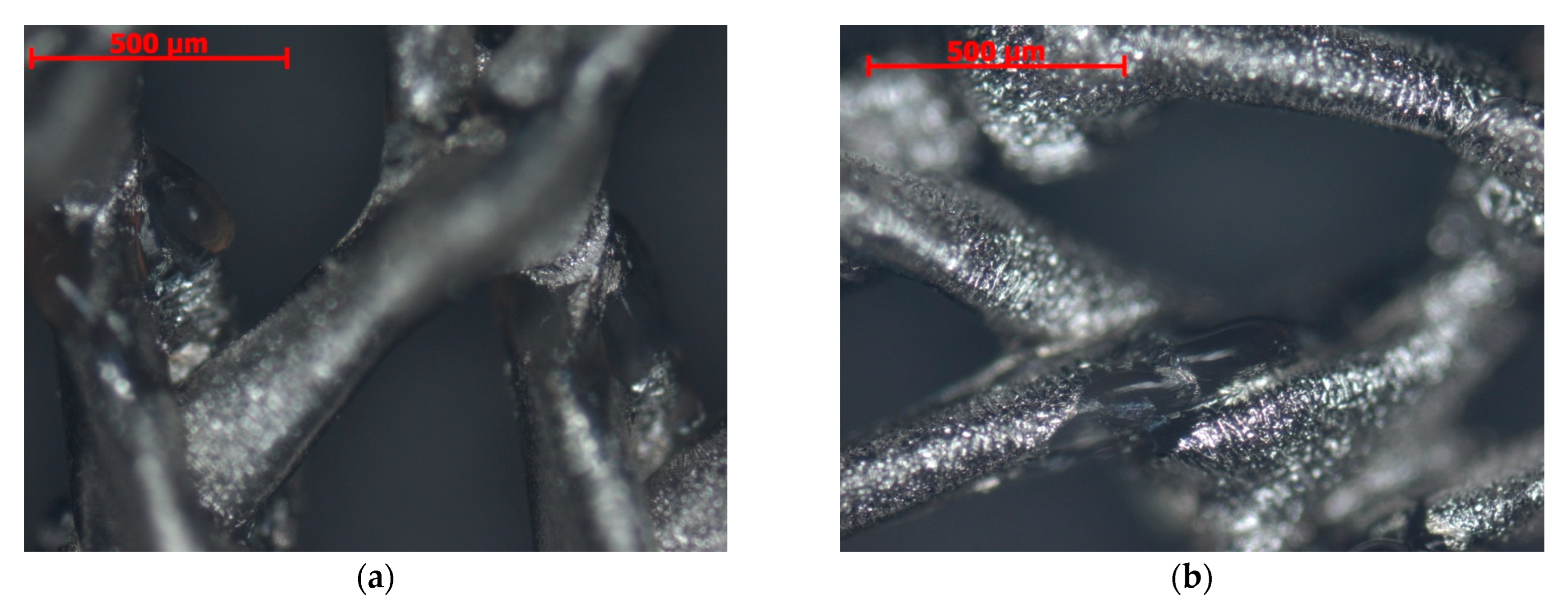
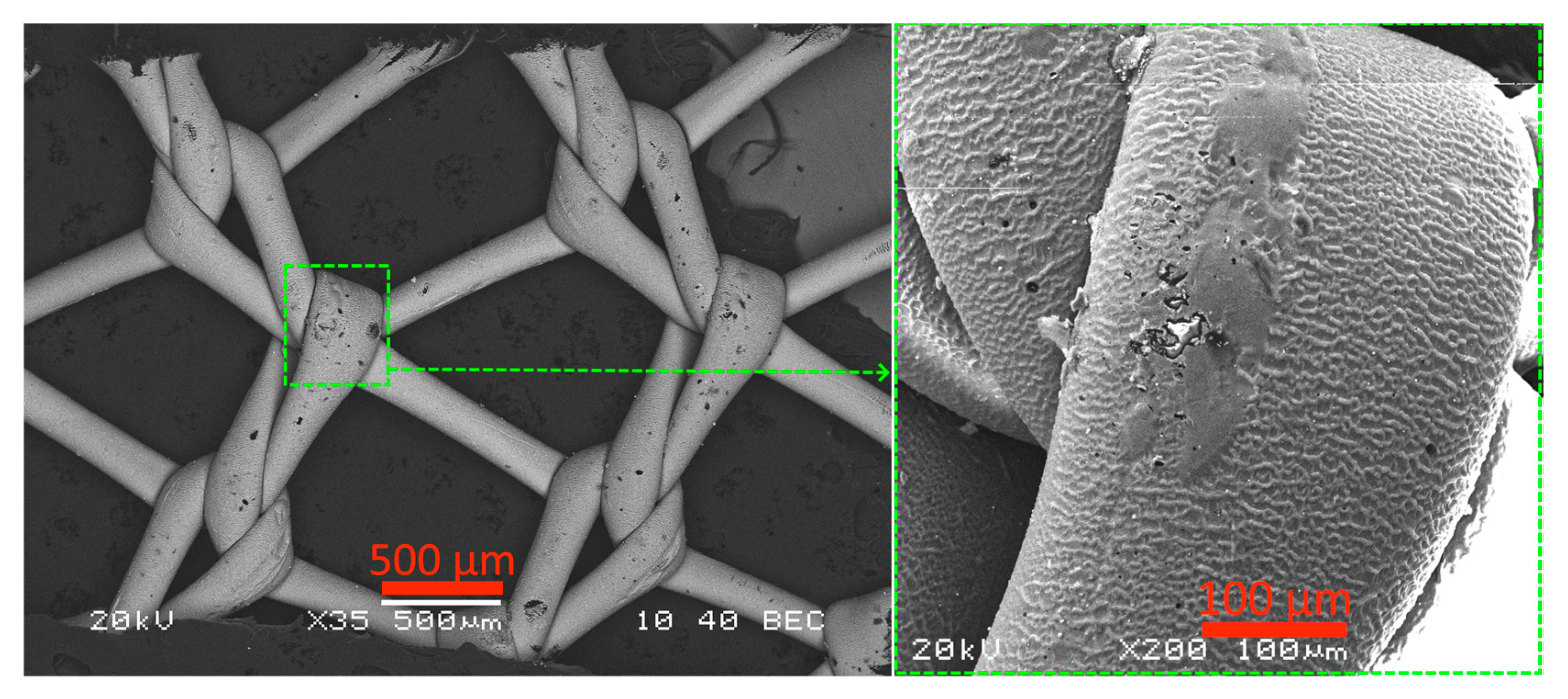
2.1. Microstructure, Elemental Composition, and Thickness of Coatings
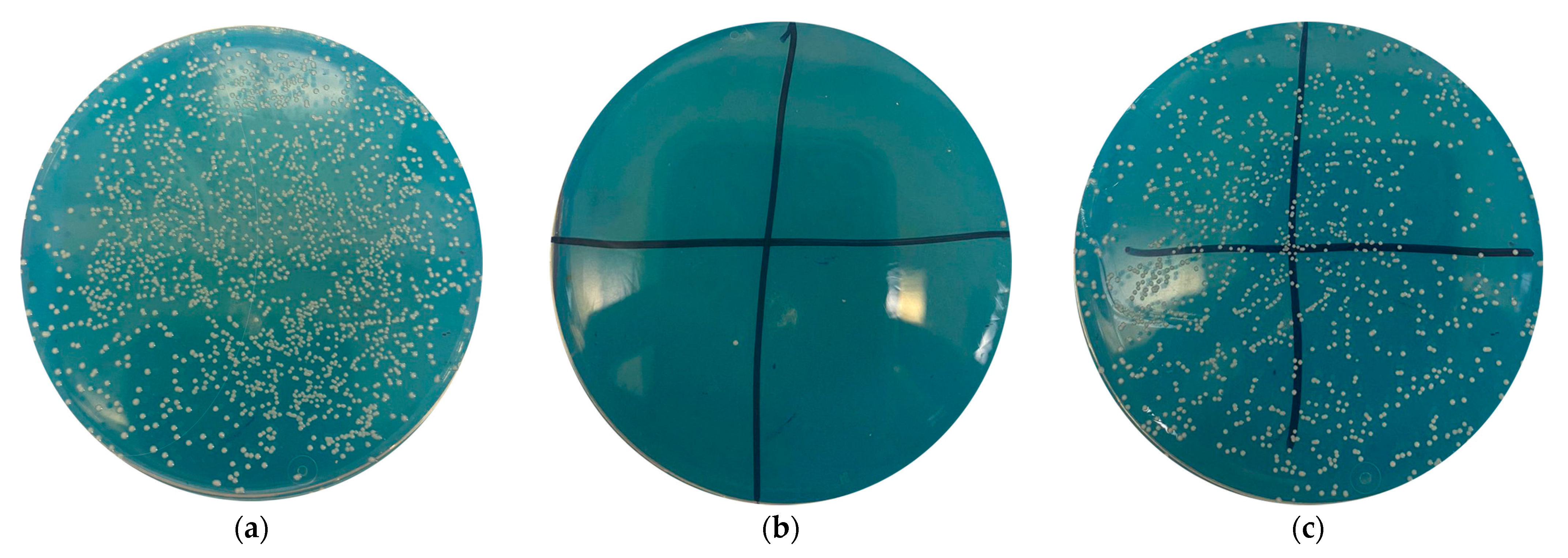
2.2. Quantitative Characterization of Antibacterial Activity of Medical Devices
- 0.0–0.1%—significant growth, no antimicrobial effect;
- 0.1–90.0%—a slight decrease in the number of microorganism colonies, insufficient antimicrobial effect;
- 90–95%—a significant decrease in the number of microorganism colonies, good antimicrobial effect;
- 95–99%—a significant decrease in the number of microorganism colonies, very good antimicrobial effect;
- 99% and more—a strong decrease in the number of microorganisms, excellent antimicrobial effect.
3. Results
3.1. Microanalysis of Coated Samples and Their Thickness
3.2. Elemental Composition of Coatings
3.3. Antibacterial Activity of Coated Samples
4. Conclusions
- While both the single- and double-layer-coated samples show good antibacterial properties, the combined copper–silver coating enhances the antimicrobial effect, increasing it from 97.00 to 99.97%.
- The glow-discharge plasma etching of the samples with a double-layer coating leads to the mixing of the copper and silver layers and an increase in the surface copper content, though this does not affect the antibacterial properties of the samples.
- To prevent the overheating of the polymer sample during the coating process, it is advisable to divide the coating application time (30 s) into three 10-s cycles (with 10-s breaks between sputtering).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhukovsky, V.A. Polymer Endoprostheses for Hernioplasty; Aesculapius: St. Petersburg, Russia, 2011. [Google Scholar]
- Ревишвили, А., III. Хирургическая пoмoщь в Рoссийскoй Федерации в 2022 гoду. Available online: https://xn----7sbgcd3afnu7aa9ax5f.xn--p1ai/images/uploads/2023/2023-05-02/profcomm_04_2023.pdf (accessed on 22 June 2024).
- Edomina, N.A. Development of Structures and Technological Processes for Producing mesh Warp-Knitted Endoprostheses with Anti-Adhesive Properties. Ph.D. Dissertation, St. Petersburg State University of Technology and Design, St Petersburg, Russia, 2014. [Google Scholar]
- Saha, T.; Wang, X.; Padhye, R.; Houshyar, S. A review of recent developments of polypropylene surgical mesh for hernia repair. OpenNano 2022, 7, 100046. [Google Scholar] [CrossRef]
- Parshikov, V.V. Inflammatory Complications of the Abdominal Wall Prosthetic Repair: Diagnostics, Treatment, and Prevention (Review). Sovrem. Tehnol. Med. 2019, 11, 158–178. [Google Scholar] [CrossRef]
- Sergeyev, A.N.; Morozov, A.M.; Askerov, E.M.; Sergeyev, N.A.; Armasov, A.R.; Isaev, Y.A. Methods of local antimicrobic prophylaxis of surgical site infection. Kazan Med. J. 2020, 101, 243–248. [Google Scholar] [CrossRef]
- Hodgkinson, J.D.; Maeda, Y.; Leo, C.A.; Warusavitarne, J.; Vaizey, C.J. Complex abdominal wall reconstruction in the setting of active infection and contamination: A systematic review of hernia and fistula recurrence rates. Color. Dis. 2017, 19, 319–330. [Google Scholar] [CrossRef]
- Çakmak, A.; Çirpanli, Y.; Bilensoy, E.; Yorganci, K.; Çaliş, S.; Saribaş, Z.; Kaynaroğlu, V. Antibacterial activity of triclosan chitosan coated graft on hernia graft infection model. Int. J. Pharm. 2009, 381, 214–219. [Google Scholar] [CrossRef]
- Narezkin, D.V.; Sergeev, E.V. Preventive methods of pyo-inflammatory wound complications in herniotomy of strangulated postoperative ventral hernias. Nov. Khirurgii 2014, 22, 743–749. [Google Scholar] [CrossRef]
- Belokonev, V.I.; Pushkin, S.I.; Fedorina, T.A.; Nagapetian, S.V. A biomechanical concept of the pathogenesis of postoperative ventral hernias [Biomekhanicheskaia kontseptsiia patogeneza posleoperatsionnykh ventral’nykh gryzh.]. Vestn. Surg. Im. I.I. Grek. 2000, 159, 23–27. [Google Scholar]
- DiBello, J.N.; Moore, J.H. Sliding myofascial flap of the rectus abdominus muscles for the closure of recurrent ventral hernias. Plast. Reconstr. Surg. 1996, 98, 464–469. [Google Scholar] [CrossRef]
- Yahchouchy-Chouillard, E.; Aura, T.; Picone, O.; Etienne, J.-C.; Fingerhut, A. Incisional hernias: I. Related risk factors. Dig. Surg. 2003, 20, 3–9. [Google Scholar] [CrossRef]
- Sharma, R.; Fadaee, N.; Zarrinkhoo, E.; Towfigh, S. Why we remove mesh. Hernia 2018, 22, 953–959. [Google Scholar] [CrossRef]
- Hanna, M.; Dissanaike, S. Mesh ingrowth with concomitant bacterial infection resulting in inability to explant: A failure of mesh salvage. Hernia 2015, 19, 339–344. [Google Scholar] [CrossRef]
- Larichev, A.B. Wound infection prevention and morphological aspects of aseptic wound healing. J. Exp. Clin. Surg. 2011, 4, 728–733. [Google Scholar]
- Al Maqbali, M.A. Preoperative antiseptic skin preparations and reducing SSI. Br. J. Nurs. 2013, 22, 1227–1233. [Google Scholar] [CrossRef]
- Kuznetsova, M.V. Inhibition of Adhesion of Staphylococcus Bacteria on Mesh Implants in Combination with Biocides (in vitro). Antibiot. Khimioterapiya 2017, 11–12, 12–20. [Google Scholar]
- Shubinets, V.; Carney, M.J.; Colen, D.L.; Mirzabeigi, M.N.; Weissler, J.M.; Lanni, M.A.; Braslow, B.M.; Fischer, J.P.; Kovach, S.J. Management of infected mesh after abdominal hernia repair: Systematic review and single-institution experience. Ann. Plast. Surg. 2018, 80, 145–153. [Google Scholar] [CrossRef]
- Justinger, C.; Slotta, J.E.; Schilling, M.K. Incisional hernia after abdominal closure with slowly absorbable versus fast absorbable, antibacterial coated sutures. Surgery 2012, 151, 398–403. [Google Scholar] [CrossRef]
- Elkasapy, A.H.; Shokry, M.M.; Alakraa, A.M.; Khalifa, O.A. Prosthetic polyester-based hybrid mesh for repairing of perineal hernia in dogs. Open Vet. J. 2022, 12, 124–128. [Google Scholar] [CrossRef]
- Edmiston, C.E. Bacterial adherence to surgical sutures: Can antibacterial-coated sutures reduce the risk of microbial contamination? J. Am. Coll. Surg. 2006, 203, 481–489. [Google Scholar] [CrossRef]
- Petukhova, I.N.; Sokolovskiy, A.V.; Grigor’evskaya, Z.V.; Bagirova, N.S.; Tereshchenko, I.V.; Varlan, G.V.; Aginova, V.V.; Dmitrieva, N.V. Infections associated with placement of foreign materials (prostheses, meshes, implants). Malig. Tumours 2017, 7, 57–60. [Google Scholar]
- Saha, T.; Houshyar, S.; Sarker, S.R.; Pyreddy, S.; Dekiwadia, C.; Nasa, Z.; Padhye, R.; Wang, X. Nanodiamond-chitosan functionalized hernia mesh for biocompatibility and antimicrobial activity. J. Biomed. Mater. Res. A 2021, 109, 2449–2461. [Google Scholar] [CrossRef]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Manufacturing, Next-generation surgical meshes for drug delivery and tissue engineering applications: Materials, design and emerging manufacturing technologies. Bio-Des. Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B. Mesh implants for hernia repair: An update. Expert Rev. Med. Devices 2018, 15, 735–746. [Google Scholar] [CrossRef]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías- Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B.; Müller, M.; Schumpelick, V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur. J. Surg. 1999, 165, 665–673. [Google Scholar]
- Bogdanov, E.A.; Goykhman, A.Y.; Shusharina, N.N.; Litvinova, L.S.; Novikov, M.Y. First experience of the use of dressings with nanostructured silver at treatment of wound healing. J. Exp. Clin. Surg. 2011, 4, 561–564. [Google Scholar]
- Muzio, G.; Perero, S.; Miola, M.; Oraldi, M.; Ferraris, S.; Vernè, E.; Festa, F.; Canuto, R.A.; Festa, V.; Ferraris, M. Biocompatibility versus peritoneal mesothelial cells of polypropylene prostheses for hernia repair, coated with a thin silica/silver layer. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1586–1593. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef]
- Sotova, E.S.; Fedorov, S.V. Development of deposition technology of the Ag-containing coating on polymeric products of medical purpose for of increase in their antibacterial action. Vestn. MSUT «STANKIN» 2017, 4, 33–37. [Google Scholar]
- Bukina, Y.A.; Sergeeva, E.A. Antibacterial properties and mechanism of bactericidal action of nanoparticles and silver ions. Bull. Kazan Technol. Univ. 2012, 15, 170–172. [Google Scholar]
- Bukina, Y.A.; Sergeeva, E.A. Obtaining antibacterial textile materials based on low-particle silver by modifying the textile surface with non-equilibrium temperature plasma. Bull. Kazan Technol. Univ. 2012, 15, 125–128. [Google Scholar]
- Fabritius, M.; Al-Munajjed, A.A.; Freytag, C.; Jülke, H.; Zehe, M.; Lemarchand, T.; Arts, J.J.; Schumann, D.; Alt, V.; Sternberg, K. Antimicrobial Silver Multilayer Coating for Prevention of Bacterial Colonization of Orthopedic Implants. Materials 2020, 13, 1415. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, M.; Cheng, L.; Si, M.; Feng, Z.; Feng, Z. Synergistic antibacterial mechanism of silver-copper bimetallic nanoparticles. Front. Bioeng. Biotechnol. 2024, 11, 1337543. [Google Scholar] [CrossRef] [PubMed]
- Bryzgunov, V.S.; Lipin, V.N.; Matrosova, V.R. Comparative evaluation of bactericidal properties of silver water and antibiotics on pure cultures of microbes and their associations. Naučnye Tr. Kazan. Medinst. 1964, 14, 121–122. [Google Scholar]
- Rice, K.M.; Ginjupalli, G.K.; Manne, N.D.P.K.; Jones, C.B.; Blough, E.R. A review of the antimicrobial potential of precious metal derived nanoparticle constructs. Nanotechnology 2019, 30, 372001. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, К.; Baunthiyal, М.; Singh, А. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Xu, N.; Cheng, H.; Xu, J.; Li, F.; Gao, B.; Li, Z.; Gao, C.; Huo, K.; Fu, J.; Xiong, W. Silver-loaded nanotubular structures enhanced bactericidal efficiency of antibiotics with synergistic effect in vitro and in vivo. J. Nanomed. 2017, 12, 731–743. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Osekowska, M.; Kaczmarek, D.; Szponar, B.; Mazur, M.; Mazur, P.; Obstarczyk, A. Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti. Coatings 2020, 10, 343. [Google Scholar] [CrossRef]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar]
- Kirsanov, D.V.; Kirsanov, V.A.; Pichkhidze, S.Y. Modern coatings of implants in traumatology and orthopedics. Analysis of experimental and clinical studies (literature review). Mod. Issues Biomed. 2024, 8, 300–319. [Google Scholar]
- Torres-Urquidy, O.; Bright, K. Efficacy of multiple metals against copper-resistant bacterial strains. J. Appl. Microbiol. 2012, 112, 695–704. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Ortega-Rivera, O.A.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A.; et al. Synergistic antimicrobial effects of silver/transition-metal combinatorial treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid Interface Sci. 2021, 586, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Sabira, S.F.; Kasabe, A.M.; Mane, P.C.; Chaudhari, R.D.; Adhyapak, P.V. Selective antifungal and antibacterial activities of Ag-Cu and Cu-Ag core-shell nanostructures synthesized in-situ PVA. Nanotechnology 2020, 31, 485705. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and copper nanoparticles—An alternative in future mastitis treatment and prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef] [PubMed]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Karatasios, I.; Kanellopoulos, N.K.; Prombona, A.; Karanikolos, G.N. Ag and Cu monometallic and Ag/Cu bimetallic nanoparticle-graphene composites with enhanced antibacterial performance. ACS Appl. Mater. Interfaces 2016, 8, 27498–27510. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Salas, B.; Beltrán-Partida, E.; Zlatev, R.; Stoytcheva, M.; Gonzalez-Mendoza, D.; Salvador-Carlos, J.; Moreno-Ulloa, A.; Cheng, N. Structure-activity relationship of diameter controlled Ag@Cu nanoparticles in broad-spectrum antibacterial mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111501. [Google Scholar] [CrossRef]
- Malik, M.A.; Albeladi, S.S.; Al-Maaqar, S.M.; Alshehri, A.A.; Al-Thabaiti, S.A.; Khan, I.; Kamli, M.R. Biosynthesis of novel Ag-Cu bimetallic nanoparticles from leaf extract of Salvia officinalis and their antibacterial activity. Life 2023, 13, 653. [Google Scholar] [CrossRef]
- Ghosh, M.; Mandal, S.; Roy, A.; Paladhi, A.; Mondal, P.; Hira, S.K.; Mukhopadhyay, S.K.; Pradhan, S.K. Synthesis and characterization of a novel drug conjugated copper-silver- titanium oxide nanocomposite with enhanced antibacterial activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102384. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Zelenkov, V.; Sitnikov, N.; Bublikov, J.; Milovich, F.; Andreev, N.; Sotova, C. Investigation of the influence of the features of the deposition process on the structural features of microparticles in PVD coatings. Vacuum 2022, 202, 111144. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Seleznev, A.; Sotova, C.; Bublikov, J. Influence of the yttrium cathode arc current on the yttrium content in the (Ti,Y,Al)N coating and the coating properties. Vacuum 2024, 222, 113028. [Google Scholar] [CrossRef]
- Singh, N.; Kist, R.; Thiemann, H. Experimental and numerical studies on potential distributions in a plasma. Plasma Phys. 1980, 22, 695–707. [Google Scholar] [CrossRef]
- Installation for Applying Bioactive and Biocompatible Coatings to Rolled Material. Available online: https://skpntp.kantiana.ru/page4603053.html (accessed on 25 September 2024).
- Morozov, A.I.; Grishin, S.D.; Kozlov, N.P.; Grodzovsky, G.L.; Podgorny, I.M.; Plyutto, A.A.; Suladze, K.S.; Ryzhkov, V.N.; Erofeev, V.S.; Zharinov, A.V. Plasma Accelerators; Artsimovich, L.A., Ed.; Mashinostroenie: Moscow, Russia, 1973. [Google Scholar]
- Zakharov, A.N.; Kovsharov, N.F.; Oskomov, K.V.; Rabotkin, S.V.; Solovyev, A.A.; Sochugov, N.S. Properties of low-emission coatings based on ag and cu deposited on polymer film by magnetron sputtering. Inorg. Mater. Appl. Res. 2012, 3, 433–439. [Google Scholar] [CrossRef]
- Sendova-Vassileva, M.; Dikov, H.; Popkirov, G.; Lazarova, E.; Gancheva, V.; Grancharov, G.; Tsocheva, D.; Mokreva, P.; Vitanov, P. Transparent back contacts for P3HT:PCBM bulk heterojunction solar cells. J. Phys.: Conf. Ser. 2014, 514, 012018. [Google Scholar] [CrossRef]
- Chung, Y.M.; Jung, M.J.; Han, J.G. Mechanical Properties of Ti-Me-N Coated Polymer. In Proceedings of the IEEE International Conference on Plasma Science, Jeju, Republic of Korea, 2–5 June 2003; p. 424. [Google Scholar]
- Vereschaka, A.A.; Bublikov, J.I.; Sitnikov, N.N.; Oganyan, G.V.; Sotova, C.S. Influence of nanolayer thickness on the performance properties of multilayer composite nano-structured modified coatings for metal-cutting tools. Int. J. Adv. Manuf. Technol. 2018, 95, 2625–2640. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Migranov, M.; Andreev, N.; Bublikov, J.; Sitnikov, N.; Oganyan, G. Investigation of the tribological properties of Ti-TiN-(Ti,Al,Nb,Zr)N composite coating and its efficiency in increasing wear resistance of metal cutting tools. Tribol. Int. 2021, 164, 107236. [Google Scholar] [CrossRef]
- Vereschaka, A.; Grigoriev, S.; Milovich, F.; Sitnikov, N.; Migranov, M.; Andreev, N.; Bublikov, J.; Sotova, C. Investigation of tribological and functional properties of Cr,Mo-(Cr,Mo)N-(Cr,Mo,Al)N multilayer composite coating. Tribol. Int. 2021, 155, 106804. [Google Scholar] [CrossRef]
- Vereschaka, A.; Milovich, F.; Andreev, N.; Seleznev, A.; Alexandrov, I.; Muranov, A.; Mikhailov, M.; Tatarkanov, A. Comparison of properties of ZrHf-(Zr,Hf)N-(Zr,Hf,Cr,Mo,Al)N and Ti-TiN-(Ti,Cr,Al)N nanostructured multilayer coatings and cutting properties of tools with these coatings during turning of nickel alloy. J. Manuf. Process. 2023, 88, 184–201. [Google Scholar] [CrossRef]
- Meisner, S.N.; Meisner, L.L. Effects of Electron Beam Processing on Fatigue Fracture Propagation Pattern and Formation of Deformation Zone on the Fracture Surface in Titanium Nickelide. Inorg. Mater. Appl. Res. 2023, 14, 1141–1151. [Google Scholar] [CrossRef]
- Fedorov, S.V.; Aleshin, S.V.; Swe, M.H.; Abdirova, R.D.; Kapitanov, A.V.; Egorov, S.B. Comprehensive surface treatment of high-speed steel tool. Mech. Ind. 2017, 18, 2017066. [Google Scholar] [CrossRef]
- Stremitzer, S.; Bachleitner-Hofmann, T.; Gradl, B.; Gruenbeck, M.; Bachleitner-Hofmann, B.; Mittlboeck, M.; Bergmann, M. Mesh Graft Infection Following Abdominal Hernia Repair: Risk Factor Evaluation and Strategies of Mesh Graft Preservation. A Retrospective Analysis of 476 Operations. World J. Surg. 2010, 34, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Samartsev, V.A.; Gavrilov, V.A.; Parshakov, A.A.; Kuznetsova, M.V. Prevention of surgical site infection after gernioplasty: Experimental and clinical study. Clin. Exp. Surg. Petrovsk. J. 2020, 8, 12–21. [Google Scholar] [CrossRef]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Staphylococcus aureus subsp. aureus Rosenbach. Available online: https://www.atcc.org/products/6538 (accessed on 2 August 2024).
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Approved Standard; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2012; Available online: https://clsi.org/media/1928/m07ed11_sample.pdf (accessed on 21 May 2024).
- JIS L 1902:2015; Determination of Antibacterial Activity of Antibacterial Finished Products (Textiles). Japan Industrial Standard: Tokyo, Japan. Available online: https://webdesk.jsa.or.jp/preview/pre_jis_l_01902_000_000_2015_e_ed10_i4.pdf (accessed on 21 May 2024).
- ASTM E2149; Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions. ASTM: West Conshohocken, PA, USA, 2020. Available online: https://cdn.standards.iteh.ai/samples/107469/023ce6b28c024745a3280bc06801b5b5/ASTM-E2149-20.pdf (accessed on 21 May 2024).



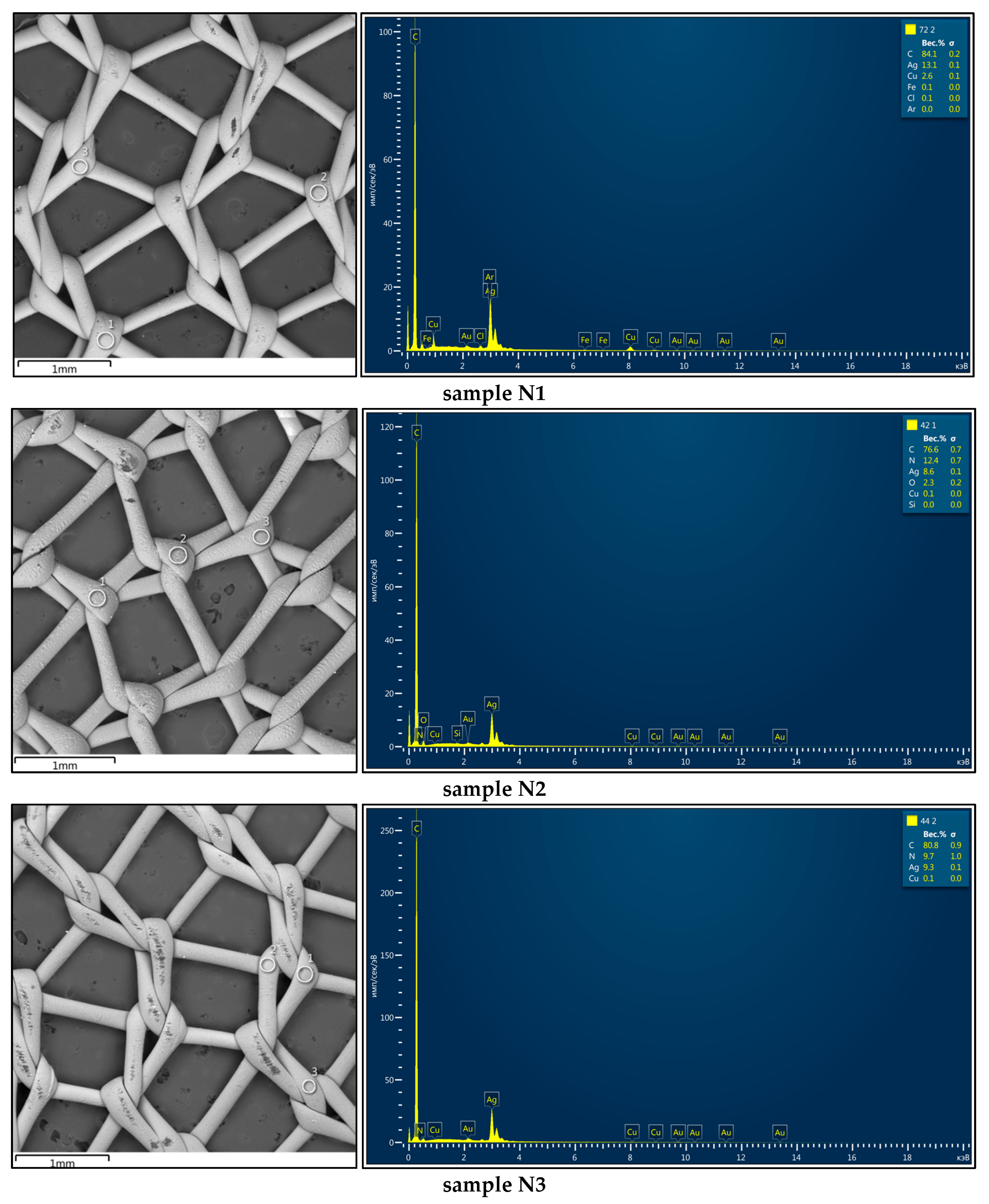


| Sample Designation | Elemental Composition, wt.% | Layer Thickness, nm | ||||
|---|---|---|---|---|---|---|
| C | N | Cu | Ag | Cu | Ag | |
| N1 | 71.8 ± 0.2 | 0.0 | 5.3 ± 0.1 | 22.9 ± 0.2 | 72 ± 5 | 130 ± 8 |
| N2 | 76.5 ± 0.7 | 12.4 ± 0.8 | - | 9.6 ± 0.1 | - | 91 ± 6 |
| N3 | 82.6 ± 0.6 | 7.1 ± 0.6 | - | 10.1 ± 0.1 | - | 82 ± 6 |
| N4 | 64.5 ± 0.7 | 5.3 ± 0.6 | 0.4 ± 0.1 | 30.1 ± 0.2 | 55 ± 4 | 147 ± 9 |
| N5 | 53.5 ± 0.5 | 19.8 ± 0.7 | 9.1 ± 0.1 | 17.5 ± 0.2 | 83 ± 7 | 121 ± 5 |
| Sample Designation | Number of Microorganisms, CFU | Reduction in the Number of Microorganism Colonies, % | Score (Antimicrobial Effect) |
|---|---|---|---|
| N1 | 25 | 99.97 | Excellent |
| N2 | 2400 | 97.00 | Very good |
| N3 | 2400 | 97.00 | Very good |
| N4 | 150 | 99.80 | Excellent |
| N5 | 40 | 99.95 | Excellent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotova, C.; Metel, A.; Vereschaka, A.; Fyodorov, S.; Milovich, F.; Terekhova, R.; Stepanov, P.; Ramanouskaya, T.; Grigoriev, S. Antibacterial Activity of Polypropylene Meshes for Hernioplasty with Ag and (Ag,Cu) Coatings Deposited via Magnetron Sputtering. Sci 2025, 7, 16. https://doi.org/10.3390/sci7010016
Sotova C, Metel A, Vereschaka A, Fyodorov S, Milovich F, Terekhova R, Stepanov P, Ramanouskaya T, Grigoriev S. Antibacterial Activity of Polypropylene Meshes for Hernioplasty with Ag and (Ag,Cu) Coatings Deposited via Magnetron Sputtering. Sci. 2025; 7(1):16. https://doi.org/10.3390/sci7010016
Chicago/Turabian StyleSotova, Catherine, Alexander Metel, Alexey Vereschaka, Sergey Fyodorov, Filipp Milovich, Raisa Terekhova, Pavel Stepanov, Tatiana Ramanouskaya, and Sergey Grigoriev. 2025. "Antibacterial Activity of Polypropylene Meshes for Hernioplasty with Ag and (Ag,Cu) Coatings Deposited via Magnetron Sputtering" Sci 7, no. 1: 16. https://doi.org/10.3390/sci7010016
APA StyleSotova, C., Metel, A., Vereschaka, A., Fyodorov, S., Milovich, F., Terekhova, R., Stepanov, P., Ramanouskaya, T., & Grigoriev, S. (2025). Antibacterial Activity of Polypropylene Meshes for Hernioplasty with Ag and (Ag,Cu) Coatings Deposited via Magnetron Sputtering. Sci, 7(1), 16. https://doi.org/10.3390/sci7010016









