Recent Advances in Microbial Enzyme Applications for Sustainable Textile Processing and Waste Management
Abstract
:1. Introduction
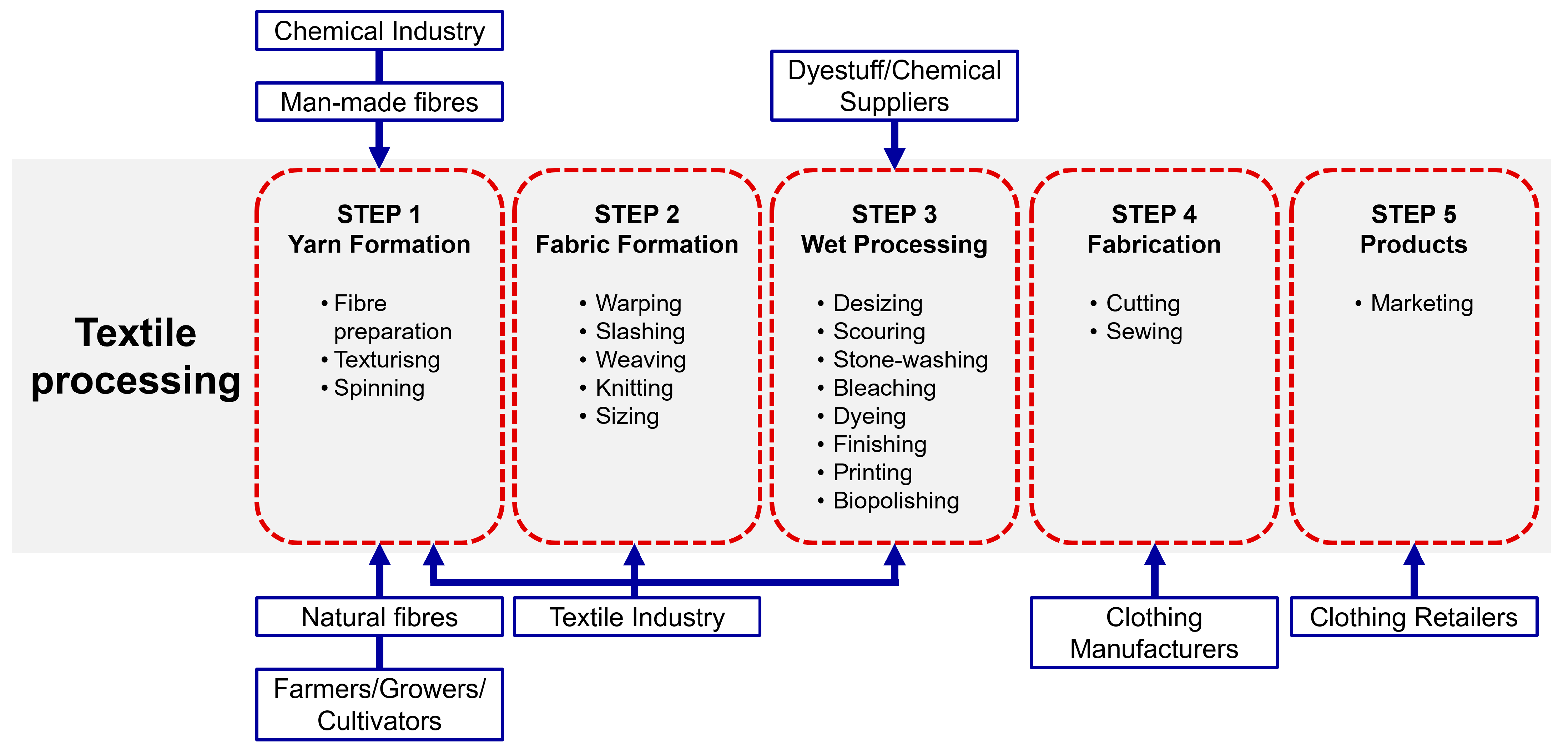
2. Textile Industrial Processes
2.1. Yarn Formation
2.2. Fabric Formation
2.3. Wet Processing
- ▪ Desizing: Removes sizing from fabric to improve absorption for dyeing and printing, often using eco-friendly enzymatic methods;
- ▪ Scouring: Removes impurities like oils and waxes, making fabric more absorbent;
- ▪ Bleaching: Whitens fabric by removing natural colours with chemicals like hydrogen peroxide (H2O2), sodium chlorite (NaClO2), or sodium hypochlorite (NaOCl), but excessive bleaching can weaken fibres;
- ▪ Mercerisation: Treats fabric with sodium hydroxide (NaOH) to improve strength, lustre, and dye uptake;
- ▪ Dyeing and Printing: Colour fabrics, with dyeing applying colour evenly and printing for specific designs. Dyeing uses more water than printing. Table 1 lists the dyes used for various synthetic and natural fabrics;
- ▪ Stone Washing: Fades colour in denim and canvas, often replaced by eco-friendly enzymatic biostoning;
- ▪ Polishing: Enhances fabric texture and brightness, often using cellulase enzymes for biopolishing.
2.4. Fabrication
2.5. Final Product
3. Textile Effluents
3.1. Microbial Remediation of Textile Effluents
3.1.1. Fungal Bioremediation
3.1.2. Bacterial Bioremediation
3.1.3. Algal Bioremediation
4. Enzymatic Innovations in Textile Processing and Effluent Treatment
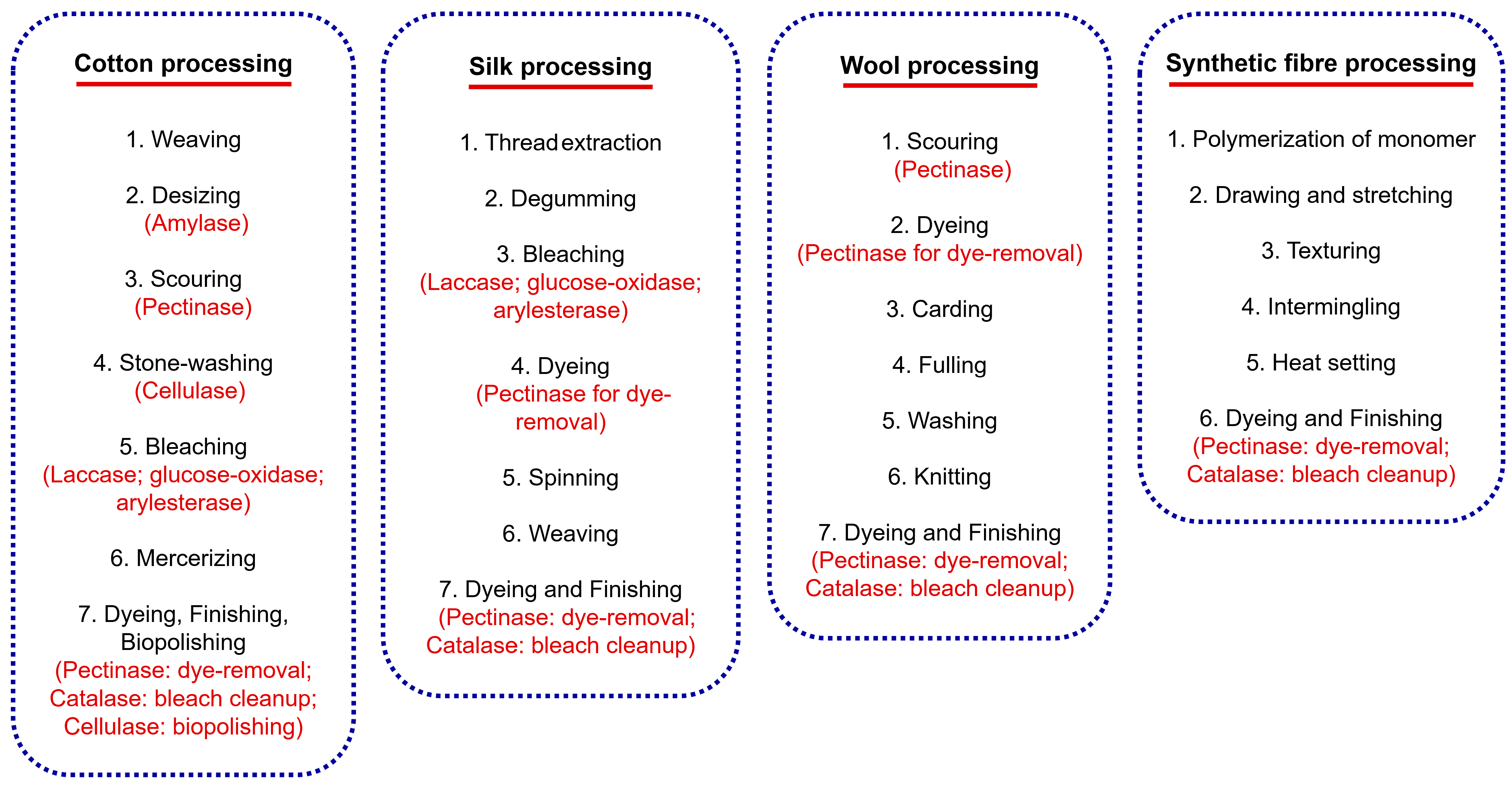
4.1. Amylases in Desizing
4.2. Pectinases in Scouring and Degumming
4.3. Cellulases in Stonewashing and Finishing
4.4. Glucose–Oxidases in Textile Bleaching
4.5. Catalase in Bleach Cleanup
4.6. Laccases in Textile Bleaching and Dye Decolourisation
4.7. Peroxidases in Dye Degradation and Effluent Treatment
4.8. Esterases and Lipases in Pre-Treatment of Textile
4.9. Arylesterases in Scouring and Bleaching
4.10. Cutinases in Surface Modification of Synthetic Fibres
4.11. Proteases in Scouring
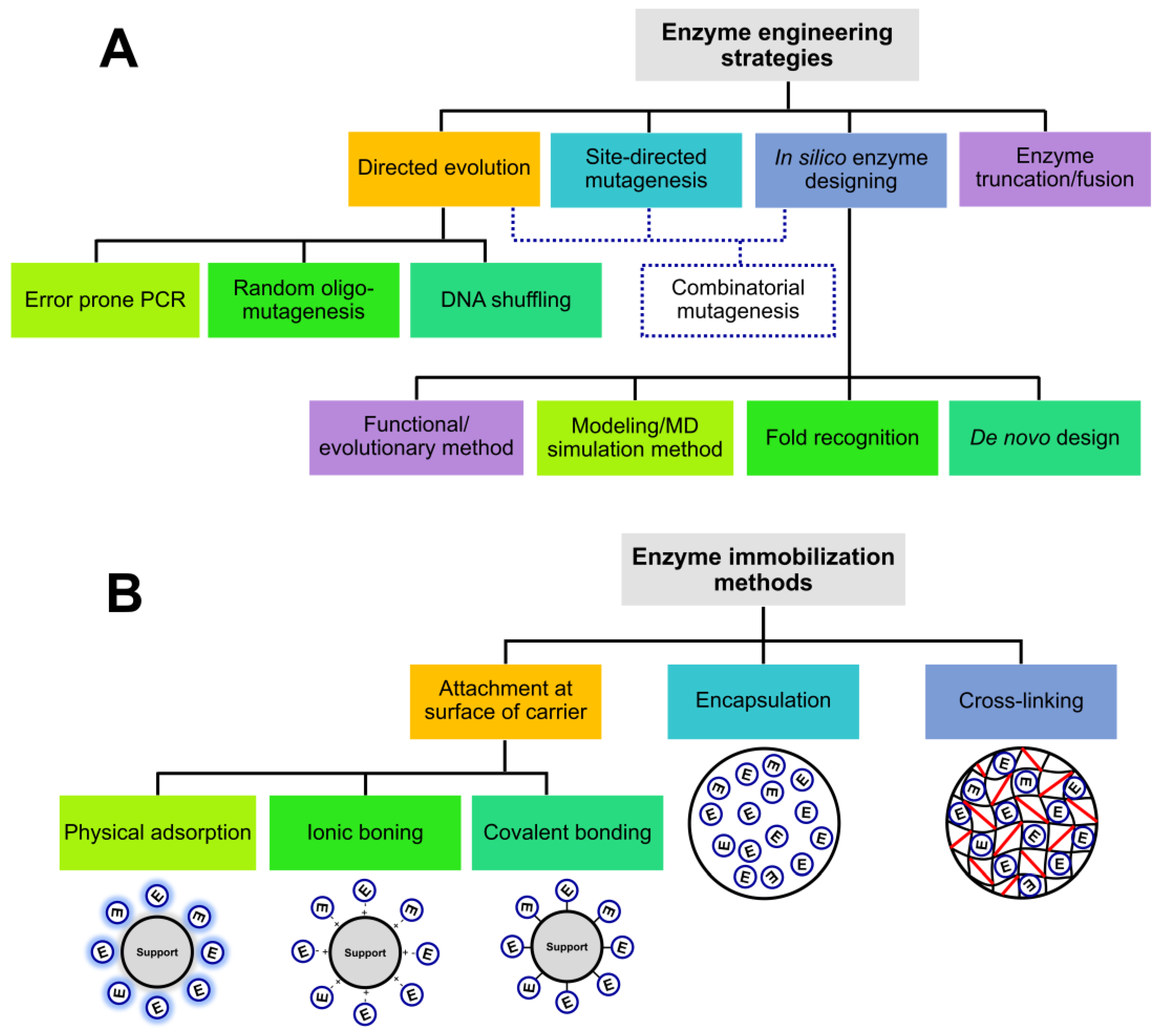
5. Improving Enzymes for Textile Applications
5.1. Recombinant and Engineered Enzymes
5.2. Immobilised Enzymes
5.3. Extremozymes—Robust Biocatalysts
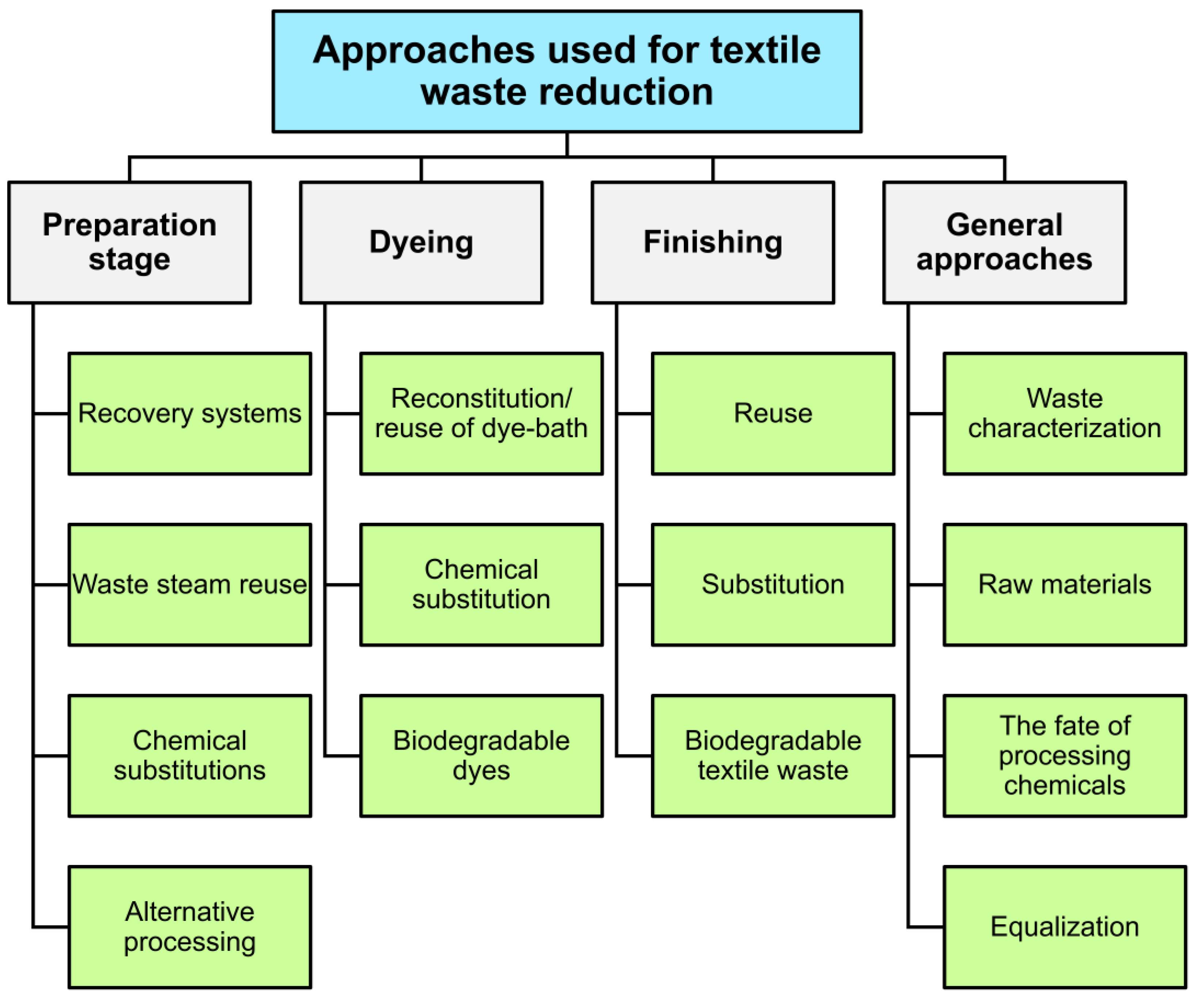
6. Strategies for Reducing Textile Waste
7. Challenges and Benefits of Enzymatic Textile Processing
8. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazotto, A.M.; de Ramos Silva, J.; de Brito, L.A.A.; Rocha, N.U.; de Souza Soares, A. How can microbiology help to improve sustainability in the fashion industry? Environ. Technol. Innov. 2021, 23, 101760. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial enzymes—An overview. In Advances in Enzyme Technology; Pandey, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Kumar, D.; Bhardwaj, R.; Jassal, S.; Goyal, T.; Khullar, A.; Gupta, N. Application of enzymes for an eco-friendly approach to textile processing. Environ. Sci. Pollut. Res. 2021, 30, 71838–71848. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Sen, A.; Kapila, R.; Chaudhary, S.; Nigam, A. Biotechnological applications of microbial enzymes to replace chemicals in the textile industry—A review. Text. Assoc. 2021, 82, 68–73. [Google Scholar]
- Sujitha, P.; Kavitha, S.; Shakilanishi, S.; Babu, N.K.C.; Shanthi, C. Enzymatic dehairing: A comprehensive review on the mechanistic aspects with emphasis on enzyme specificity. Int. J. Biol. Macromol. 2018, 118, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Bernal, S.P.; Lira, M.M.; Jean-Baptiste, J.; Garcia, P.E.; Batista, E.; Ottoni, J.R.; Passarini, M.R. Biotechnological potential of microorganisms from textile effluent: Isolation, enzymatic activity, and dye discolouration. An. Acad. Bras. Ciênc. 2021, 93, e20191581. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Smith, E. Enzymatic treatments for sustainable textile processing. In Sustainable Apparel; Richard, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 119–133. [Google Scholar]
- Chowdhury, I.R.; Summerscales, J. Woven fabrics for composite reinforcement: A review. J. Compos. Sci. 2024, 8, 280. [Google Scholar] [CrossRef]
- Saha, P.; Khan, M.F.; Patra, S. Truncated α-amylase: An improved candidate for textile processing. Prep. Biochem. Biotechnol. 2018, 48, 635–645. [Google Scholar] [CrossRef]
- Catarino, M.L.; Sampaio, F.; Gonçalves, A.L. Sustainable Wet Processing Technologies for the Textile Industry: A Comprehensive Review. Sustainability 2025, 17, 3041. [Google Scholar] [CrossRef]
- Singha, K.; Pandit, P.; Maity, S.; Sharma, S.R. Harmful environmental effects of textile chemical dyeing practice. In Green Chemistry for Sustainable Textiles; Muthu, S.S., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 153–164. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Fluorotelomer alcohols are efficiently biotransformed by Cunninghamella elegans. Environ. Sci. Pollut. Res. 2023, 30, 23613–23623. [Google Scholar] [CrossRef]
- Khan, M.F.; Paul Guin, J.; Thampi, R.K.; Sullivan, J.A.; Murphy, C.D. Enhanced removal of perfluorooctanoic acid with sequential photocatalysis and fungal treatment. Environ. Sci. Pollut. Res. 2023, 30, 91478–91486. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Application of microbial biofilms in biocatalysis and biodegradation. In Enzymes for Pollutant Degradation; Mulla, S.I., Bharagava, R.N., Eds.; Springer Nature Singapore: Singapore, 2022; Volume 30, pp. 93–118. [Google Scholar]
- Kuppan, N.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A comprehensive review of sustainable bioremediation techniques: Eco-friendly solutions for waste and pollution management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- Das, S.; Cherwoo, L.; Singh, R. Decoding dye degradation: Microbial remediation of textile industry effluents. Biotechnol. Notes 2023, 4, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Hof, C.; Niemcová, P.; Murphy, C.D. Biotransformation of fluorinated drugs and xenobiotics by the model fungus Cunninghamella elegans. Methods Enzymol. 2024, 696, 251–285. [Google Scholar]
- Khan, M.F.; Murphy, C.D. Bacterial degradation of the anti-depressant drug fluoxetine produces trifluoroacetic acid and fluoride ion. Appl. Microbiol. Biotechnol. 2021, 105, 9359–9369. [Google Scholar] [CrossRef]
- Pundir, A.; Thakur, M.S.; Prakash, S.; Kumari, N.; Sharma, N.; Parameswari, E.; He, Z.; Nam, S.; Thakur, M.; Puri, S.; et al. Fungi as versatile biocatalytic tool for treatment of textile wastewater effluents. Environ. Sci. Eur. 2024, 36, 185. [Google Scholar] [CrossRef]
- Rathour, R.K.; Sharma, D.; Ullah, S.; Mahmoud, E.H.M.; Sharma, N.; Kumar, P.; Bhatt, A.K.; Ahmad, I.; Bhatia, R.K. Bacterial–microalgal consortia for bioremediation of textile industry wastewater and resource recovery for circular economy. Biotechnol. Environ. 2024, 1, 6. [Google Scholar] [CrossRef]
- Sornaly, H.H.; Ahmed, S.; Titin, K.F.; Islam, M.N.; Parvin, A.; Islam, M.A.; Faruquee, H.M.; Biswas, K.K.; Islam, R.; Paul, D.K.; et al. The utility of bioremediation approach over physicochemical methods to detoxify dyes discharges from textile effluents: A comprehensive review study. Sustain. Chem. Pharm. 2024, 39, 101538. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.; Albahnasawi, A.; Ahmad, Z.; Bashir, M.J.; Al-Wahaibi, T.; Abujazar, M.S.S.; Amr, S.S.A.; Nassani, D.E. Potential use of algae for the bioremediation of different types of wastewater and contaminants: Production of bioproducts and biofuel for green circular economy. J. Environ. Manag. 2022, 324, 116415. [Google Scholar] [CrossRef]
- Singh, L.; Singh, V.P. Textile dyes degradation: A microbial approach for biodegradation of pollutants. In Microbial Degradation of Synthetic Dyes in Wastewaters; Singh, S., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 187–204. [Google Scholar]
- Latif, W.; Ciniglia, C.; Iovinella, M.; Shafiq, M.; Papa, S. Role of white rot fungi in industrial wastewater treatment: A review. Appl. Sci. 2023, 13, 8318. [Google Scholar] [CrossRef]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalysed decolourization of melanin using in situ generated H₂O₂ for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef]
- Anastasi, A.; Tigini, V.; Varese, G.C. The bioremediation potential of different ecophysiological groups of fungi. In Fungi as Bioremediators; Goltapeh, E., Danesh, Y., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–49. [Google Scholar]
- Corso, C.R.; Almeida, E.J.R.; Santos, G.C.; Morão, L.G.; Fabris, G.S.L.; Mitter, E.K. Bioremediation of direct dyes in simulated textile effluents by a paramorphogenic form of Aspergillus oryzae. Water Sci. Technol. 2012, 65, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Hof, C.; Niemcová, P.; Murphy, C.D. Recent advances in fungal xenobiotic metabolism: Enzymes and applications. World J. Microbiol. Biotechnol. 2023, 39, 296. [Google Scholar] [CrossRef]
- Rao, P.; Prathibha, N.; Birawat, K.K.; Kankrej, S.; Nayak, S.; Varsha, N. Decolourisation of synthetic dyes using Aspergillus species. J. Chem. Eng. Res. 2014, 2, 61–68. [Google Scholar]
- Aragaw, T.A. A review on biodegradation of textile dye wastewater: Challenges due to wastewater characteristics and the potential of alkaliphiles. J. Hazard. Mater. Adv. 2024, 5, 100117. [Google Scholar] [CrossRef]
- Mustafa, G.; Zahid, M.T.; Ali, S.; Abbas, S.Z.; Rafatullah, M. Biodegradation and discolouration of disperse blue-284 textile dye by Klebsiella pneumoniae GM-04 bacterial isolate. J. King Saud Univ.-Sci. 2021, 33, 101442. [Google Scholar] [CrossRef]
- Ameenudeen, S.; Unnikrishnan, S.; Ramalingam, K. Statistical optimization for the efficacious degradation of reactive azo dyes using Acinetobacter baumannii JC359. J. Environ. Manag. 2021, 279, 111512. [Google Scholar] [CrossRef]
- Kishor, R.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Iqbal, H.M.; Bharagava, R.N. Efficient degradation and detoxification of methylene blue dye by a newly isolated ligninolytic enzyme producing bacterium Bacillus albus MW407057. Colloids Surf. B Biointerfaces 2021, 206, 111947. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Fu, C.C.; Juang, R.S. Biosorption and biodegradation of a sulfur dye in high-strength dyeing wastewater by Acidithiobacillus thiooxidans. J. Environ. Manag. 2016, 182, 265–271. [Google Scholar] [CrossRef]
- Bera, S.P.; Tank, S.K. Microbial degradation of Procion Red by Pseudomonas stutzeri. Sci. Rep. 2021, 11, 3075. [Google Scholar] [CrossRef] [PubMed]
- Lade, H.; Kadam, A.; Paul, D.; Govindwar, S. Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J. 2015, 14, 158. [Google Scholar]
- HP, J.S.P.; Girish, K.; Agsar, D. Optimization of process conditions for the effective biodegradation of azo orange dye by actinomycetes. Indian J. Nat. Sci. 2015, 5, 5248–5258. [Google Scholar]
- Bharagava, R.N.; Mani, S.; Mulla, S.I.; Saratale, G.D. Degradation and decolourization potential of a ligninolytic enzyme producing Aeromonas hydrophila for crystal violet dye and its phytotoxicity evaluation. Ecotoxicol. Environ. Saf. 2018, 156, 166–175. [Google Scholar] [CrossRef]
- Sharma, N.; Chatterjee, S.; Bhatnagar, P. Degradation of Direct Red 28 by Alcaligenes sp. TEX S6 isolated from aeration tank of Common Effluent Treatment Plant (CETP), Pali, Rajasthan. Nat. Environ. Pollut. Technol. 2019, 18, 9–20. [Google Scholar]
- Oturkar, C.C.; Patole, M.S.; Gawai, K.R.; Madamwar, D. Enzyme-based cleavage strategy of Bacillus lentus BI377 in response to metabolism of azoic recalcitrant. Bioresour. Technol. 2013, 130, 360–365. [Google Scholar] [CrossRef]
- Ewida, A.Y.; El-Sesy, M.E.; Abou Zeid, A. Complete degradation of azo dye acid red 337 by Bacillus megaterium KY848339.1 isolated from textile wastewater. Water Sci. 2019, 33, 154–161. [Google Scholar] [CrossRef]
- Amin, S.; Rastogi, R.P.; Chaubey, M.G.; Jain, K.; Divecha, J.; Desai, C.; Madamwar, D. Degradation and toxicity analysis of a reactive textile diazo dye-Direct Red 81 by newly isolated Bacillus sp. DMS2. Front. Microbiol. 2020, 11, 576680. [Google Scholar] [CrossRef]
- Dixit, S.; Garg, S. Enzymatic degradation of sulphonated azo dye using purified azoreductase from facultative Klebsiella pneumoniae. Folia Microbiol. 2021, 66, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.A.; Satyanarayana, V.S.V.; Rao, K.B. Biotransformation of Direct Blue 1 by a moderately halophilic bacterium Marinobacter sp. strain HBRA and toxicity assessment of degraded metabolites. J. Hazard. Mater. 2013, 262, 674–684. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.; Khalid, A.; Hussain, S.; Mumtaz, M.W.; Crowley, D.E. Decolourization of Reactive Black-5 by Shewanella sp. in the presence of metal ions and salts. Water Environ. Res. 2015, 87, 579–586. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.Y.; Tang, Q.W.; Li, J.; Xie, T.; Liu, C.; Cao, M.Y.; Zhang, R.C.; Wang, S.; Hu, J.M.; et al. Decolourization characteristics of a newly isolated salt-tolerant Bacillus sp. strain and its application for azo dye-containing wastewater in immobilised form. Appl. Microbiol. Biotechnol. 2015, 99, 9277–9287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, L.; Li, H.; Wang, Y.; Chen, G.; Zhang, Q. Biodegradation and detoxification of Direct Black G textile dye by a newly isolated thermophilic microflora. Bioresour. Technol. 2018, 250, 650–657. [Google Scholar] [CrossRef]
- Rathod, J.; Dhebar, S.; Archana, G. Efficient approach to enhance whole cell azo dye decolourization by heterologous overexpression of Enterococcus sp. L2 azoreductase (azoA) and Mycobacterium vaccae formate dehydrogenase (fdh) in different bacterial systems. Int. Biodeterior. Biodegrad. 2017, 124, 91–100. [Google Scholar] [CrossRef]
- Balapure, K.; Aghera, P.; Bhatt, N.; Madamwar, D. Community synergism: Degradation of triazine dye reactive black 1 by mixed bacterial cultures KND_PR under microaerophilic and aerobic conditions. Environ. Process. 2019, 6, 713–739. [Google Scholar] [CrossRef]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae- and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Alvarez, M.S.; Rodriguez, A.; Sanroman, M.A.; Deive, F.J. Microbial adaptation to ionic liquids. RSC Adv. 2015, 5, 17379–17382. [Google Scholar] [CrossRef]
- Devi, S.; Murugappan, A.; Kannan, R.R. Sorption of Reactive Blue 19 onto freshwater algae and seaweed. Desalination Water Treat. 2015, 54, 2611–2624. [Google Scholar] [CrossRef]
- Thirumagal, J.; Panneerselvam, A. Isolation of azoreductase enzyme in its various forms from Chlorella pyrenoidosa and its immobilisation efficiency for treatment of water. Int. J. Sci. Res. 2016, 5, 2133–2138. [Google Scholar]
- Dellamatrice, P.M.; Silva-Stenico, M.E.; Moraes, L.A.B.D.; Fiore, M.F.; Monteiro, R.T.R. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2017, 48, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Sharma, M.; Bala, S.; Thakur, V.K.; Singh, A.; Dashora, K.; Hart, P.; Gupta, V.K. Recent technologies for transforming textile waste into value-added products: A review. Curr. Res. Biotechnol. 2024, 7, 100225. [Google Scholar] [CrossRef]
- Ahmed, T.; Mia, R.; Toki, G.F.I.; Jahan, J.; Hasan, M.M.; Tasin, M.A.S.; Farsee, M.S.; Ahmed, S. Evaluation of sizing parameters on cotton using the modified sizing agent. Clean. Eng. Technol. 2021, 5, 100320. [Google Scholar] [CrossRef]
- Colombi, B.L.; Valle, R.D.; Valle, J.A.; Andreaus, J. Advances in sustainable enzymatic scouring of cotton textiles: Evaluation of different post-treatments to improve fabric wettability. Clean. Eng. Technol. 2021, 4, 100160. [Google Scholar] [CrossRef]
- Islam, M.T.; Huda, S.Z.; Alam, M.S.; Sahariar, M.F. Single-bath-single-stage enzymatic treatment of denim. Results Eng. 2024, 21, 101944. [Google Scholar] [CrossRef]
- Farooq, A.; Ali, S.; Abbas, N.; Fatima, G.A.; Ashraf, M.A. Comparative performance evaluation of conventional bleaching and enzymatic bleaching with glucose oxidase on knitted cotton fabric. J. Clean. Prod. 2013, 42, 167–171. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M.; Tesfaye, E.L. Oxidative ligninolytic enzymes and their role in textile dye biodegradation: A comprehensive review. Water Pract. Technol. 2024, 19, 3598–3630. [Google Scholar] [CrossRef]
- Paar, A.; Costa, S.; Tzanov, T.; Gudelj, M.; Robra, K.H.; Cavaco-Paulo, A.; Gübitz, G.M. Thermo-alkali-stable catalases from newly isolated Bacillus sp. for the treatment and recycling of textile bleaching effluents. J. Biotechnol. 2001, 89, 147–153. [Google Scholar] [CrossRef]
- Gautam, R.L.; Bharadwaj, A.K.; Kumar, S.; Naraian, R. Microbial enzymes for the variable applications of textile industry processing. In Valorization of Biomass to Bioproducts; Vijai, K.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 3, pp. 297–321. [Google Scholar]
- Ramasubbu, N.; Paloth, V.; Luo, Y.; Brayer, G.D.; Levine, M.J. Structure of human salivary α-amylase at 1.6 Å resolution: Implications for its role in the oral cavity. Acta Crystallogr. Sect. D Biol. Crystallogr. 1996, 52, 435–446. [Google Scholar] [CrossRef]
- Far, B.E.; Ahmadi, Y.; Khosroshahi, A.Y.; Dilmaghani, A. Microbial alpha-amylase production: Progress, challenges and perspectives. Adv. Pharm. Bull. 2020, 10, 350. [Google Scholar]
- Ali, Z.; Abdullah, M.; Yasin, M.T.; Amanat, K.; Sultan, M.; Rahim, A.; Sarwar, F. Recent Trends in Production and potential applications of microbial amylases: A comprehensive review. Protein Expr. Purif. 2024, 227, 106640. [Google Scholar] [CrossRef]
- Rehman, A.; Saeed, A.; Asad, W.; Khan, I.; Hayat, A.; Rehman, M.U.; Shah, T.A.; Sitotaw, B.; Dawoud, T.M.; Bourhia, M. Eco-friendly textile desizing with indigenously produced amylase from Bacillus cereus AS2. Sci. Rep. 2023, 13, 11991. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.; Bali, V.; Sharma, L.; Mangla, J. Production of fungal amylases using cheap, readily available agri-residues, for potential application in textile industry. BioMed Res. Int. 2014, 2014, 215748. [Google Scholar] [CrossRef]
- Hao, L.; Wang, R.; Fang, K.; Liu, J. Ultrasonic effect on the desizing efficiency of α-amylase on starch-sized cotton fabrics. Carbohydr. Polym. 2013, 96, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Sajedi, R.H.; Naderi-Manesh, H.; Khajeh, K.; Ahmadvand, R.; Ranjbar, B.; Asoodeh, A.; Moradian, F. A Ca-independent α-amylase that is active and stable at low pH from the Bacillus sp. KR-8104. Enzym. Microb. Technol. 2005, 36, 666–671. [Google Scholar] [CrossRef]
- Chand, N.; Sajedi, R.H.; Nateri, A.S.; Khajeh, K.; Rassa, M. Fermentative desizing of cotton fabric using an α-amylase-producing Bacillus strain: Optimization of simultaneous enzyme production and desizing. Process Biochem. 2014, 49, 1884–1888. [Google Scholar] [CrossRef]
- Zafar, A.; Aftab, M.N.; Iqbal, I.; ud Din, Z.; Saleem, M.A. Pilot-scale production of a highly thermostable α-amylase enzyme from Thermotoga petrophila cloned into E. coli and its application as a desizer in textile industry. RSC Adv. 2019, 9, 984–992. [Google Scholar] [CrossRef]
- Garg, G.; Singh, A.; Kaur, A.; Singh, R.; Kaur, J.; Mahajan, R. Microbial pectinases: An ecofriendly tool of nature for industries. 3 Biotech 2016, 6, 47. [Google Scholar] [CrossRef]
- Madhu, A.; Chakraborty, J.N. Developments in application of enzymes for textile processing. J. Clean. Prod. 2017, 145, 114–133. [Google Scholar] [CrossRef]
- Haile, S.; Ayele, A. Pectinase from microorganisms and its industrial applications. Sci. World J. 2022, 2022, 1881305. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Bhatti, H.N.; Bilal, M. Recent advances in the production strategies of microbial pectinases—A review. Int. J. Biol. Macromol. 2019, 122, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Debing, J.; Peijun, L.; Stagnitti, F.; Xianzhe, X.; Li, L. Pectinase production by solid fermentation from Aspergillus niger by a new prescription experiment. Ecotoxicol. Environ. Saf. 2006, 64, 244–250. [Google Scholar] [CrossRef]
- Vigneswaran, C.; Anbumani, N.; Ananthasubramanian, M.; Rajendran, R. Prediction and process optimization of pectinolytic reaction on organic cotton fabrics for bioscouring with alkaline pectinase. Indian J. Fibre Text. Res. 2012, 37, 183–190. [Google Scholar]
- Rajendran, R.; Sundaram, S.K.; Radhai, R.; Rajapriya, P. Bioscouring of cotton fabrics using pectinase enzyme its optimization and comparison with conventional scouring process. Pak. J. Biol. Sci. 2011, 14, 519–525. [Google Scholar] [CrossRef]
- Guo, F.; Zou, M.; Li, X.; Zhao, J.; Qu, Y. An effective degumming enzyme from Bacillus sp. Y1 and synergistic action of hydrogen peroxide and protease on enzymatic degumming of ramie fibers. BioMed Res. Int. 2013, 2013, 212315. [Google Scholar] [CrossRef]
- Zheng, L.; Du, Y.; Zhang, J. Degumming of ramie fibers by alkalophilic bacteria and their polysaccharide-degrading enzymes. Bioresour. Technol. 2001, 78, 89–94. [Google Scholar] [CrossRef]
- Degani, O. Synergism between cutinase and pectinase in the hydrolysis of cotton fibers’ cuticle. Catalysts 2021, 11, 84. [Google Scholar] [CrossRef]
- Hebeish, A.; Hashem, M.; Shaker, N.; Ramadan, M.; El-Sadek, B.; Hady, M.A. New development for combined bioscouring and bleaching of cotton-based fabrics. Carbohydr. Polym. 2009, 78, 961–972. [Google Scholar] [CrossRef]
- Špička, N.; Tavčer, P.F. Complete enzymatic pre-treatment of cotton fabric with incorporated bleach activator. Text. Res. J. 2013, 83, 566–573. [Google Scholar] [CrossRef]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef]
- Pei, J.; Pang, Q.; Zhao, L.; Fan, S.; Shi, H. Thermoanaerobacterium thermosaccharolyticum β-glucosidase: A glucose-tolerant enzyme with high specific activity for cellobiose. Biotechnol. Biofuels 2012, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Sutaoney, P.; Rai, S.N.; Sinha, S.; Choudhary, R.; Gupta, A.K.; Singh, S.K.; Banerjee, P. Current perspective in research and industrial applications of microbial cellulases. Int. J. Biol. Macromol. 2024, 264, 130639. [Google Scholar] [CrossRef]
- Elfaleh, I.; Abbassi, F.; Habibi, M.; Ahmad, F.; Guedri, M.; Nasri, M.; Garnier, C. A comprehensive review of natural fibers and their composites: An eco-friendly alternative to conventional materials. Results Eng. 2023, 19, 101271. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; More, V.S.; Nsanganwimana, F.; Rao, A.S.; Nair, A.; Anantharaju, K.S.; More, S.S. Microbial enzymes used in textile industry. In Biotechnology of Microbial Enzymes, 2nd ed.; Academic Press: London, UK, 2023; pp. 649–684. [Google Scholar]
- Maryan, A.S.; Montazer, M. A cleaner production of denim garment using one step treatment with amylase/cellulase/laccase. J. Clean. Prod. 2013, 57, 320–326. [Google Scholar] [CrossRef]
- Jayasekara, S.; Ratnayake, R. Microbial cellulases: An overview and applications. Cellulose 2019, 22, 10–5772. [Google Scholar]
- Choudhury, A.K.R. Various ecofriendly finishes. Principles of Textile Finishing, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 467–525. [Google Scholar]
- Lenin, V.; Kandasamy, N.; Karthick, S.; Kanipriya, M. Enzymes in Textile Finishing. Man-Made Text. India 2009, 52, 7. [Google Scholar]
- Araujo, R.; Casal, M.; Cavaco-Paulo, A. Application of enzymes for textile fibres processing. Biocatal. Biotransformation 2008, 26, 332–349. [Google Scholar] [CrossRef]
- Korsa, G.; Konwarh, R.; Masi, C.; Ayele, A.; Haile, S. Microbial cellulase production and its potential application for textile industries. Ann. Microbiol. 2023, 73, 13. [Google Scholar] [CrossRef]
- Walawska, A.; Olak-Kucharczyk, M.; Kaczmarek, A.; Kudzin, M.H. Environmentally friendly bleaching process of the cellulose fibres materials using ozone and hydrogen peroxide in the gas phase. Materials 2024, 17, 1355. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, S. Microbial enzyme in food biotechnology. In Enzymes in Food Biotechnology, 2nd ed.; Academic Press: London, UK, 2019; pp. 19–28. [Google Scholar]
- Tochetto, G.A.; Aragão, A.M.; de Oliveira, D.; Immich, A.P. Can enzymatic processes transform textile processes? A critical analysis of the industrial application. Process Biochem. 2022, 123, 27–35. [Google Scholar] [CrossRef]
- Davulcu, A.; Eren, H.A.; Avinc, O.; Erişmiş, B. Ultrasound assisted biobleaching of cotton. Cellulose 2014, 21, 2973–2981. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; El-Shafie, A.; El Zawahry, M.M. Ecofriendly laccase–hydrogen peroxide/ultrasound-assisted bleaching of linen fabrics and its influence on dyeing efficiency. Ultrason. Sonochem. 2010, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, D.; Sivasaravanan, S.; Sudharshan Prabhu, M.; Vasanthi, N.S.; Senthil Raja, K.; Das, A.; Ramachandran, T. One-step process for desizing and bleaching of cotton fabrics using the combination of amylase and glucose oxidase enzymes. J. Appl. Polym. Sci. 2012, 123, 2445–2450. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; You, L.; Osuji, C.M.; Iwouno, J.O.; Kabuo, N.O.; Ojukwu, M.; Agunwah, I.M.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Hydrogen peroxide effects on natural-sourced polysaccharides: Free radical formation/production, degradation process, and reaction mechanism—A critical synopsis. Foods 2021, 10, 699. [Google Scholar] [CrossRef]
- Mojsov, K. Enzymatic desizing, bioscouring and enzymatic bleaching of cotton fabric with glucose oxidase. J. Text. Inst. 2019, 110, 1032–1041. [Google Scholar] [CrossRef]
- Konczewicz, W.; Kozłowski, R.M. Enzymatic treatment of natural fibres. In Handbook of Natural Fibres, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 168–184. [Google Scholar]
- Fu, S.; Farrell, M.J.; Ankeny, M.A.; Turner, E.T.; Rizk, V. Hydrogen peroxide bleaching of cationized cotton fabric. AATCC J. Res. 2019, 6, 21–29. [Google Scholar] [CrossRef]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring therapeutic potential of catalase: Strategies in disease prevention and management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Sooch, B.S.; Kauldhar, B.S.; Puri, M. Isolation and polyphasic characterization of a novel hyper catalase producing thermophilic bacterium for the degradation of hydrogen peroxide. Bioprocess Biosyst. Eng. 2016, 39, 1759–1773. [Google Scholar] [CrossRef]
- Czyzewska, K.; Trusek-Holownia, A.; Dabrowa, M.; Sarmiento, F.; Blamey, J.M. A catalytic membrane used for H2O2 decomposition. Catal. Today 2019, 331, 30–34. [Google Scholar] [CrossRef]
- Shaeer, A.; Aslam, M.; Rashid, N. A highly stable manganese catalase from Geobacillus thermopakistaniensis: Molecular cloning and characterization. Extremophiles 2019, 23, 707–718. [Google Scholar] [CrossRef]
- Shaeer, A.; Aroob, I.; Aslam, M.; Azim, N.; Rashid, N. Investigating recombinant manganese-catalases from Geobacillus thermopakistaniensis for sustainable and eco-friendly textile processing. Int. J. Environ. Sci. Technol. 2024, 22, 6903–6912. [Google Scholar] [CrossRef]
- Lončar, N.; Fraaije, M.W. Catalases as biocatalysts in technical applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 3351–3357. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, N. Microbial laccase: A robust enzyme and its industrial applications. Biologia 2020, 75, 1183–1193. [Google Scholar] [CrossRef]
- Mojsov, K. Biotechnological applications of laccases in the textile industry. Adv. Technol. 2014, 3, 76–79. [Google Scholar] [CrossRef]
- Kim, S.; Moldes, D.; Cavaco-Paulo, A. Laccases for enzymatic colouration of unbleached cotton. Enzym. Microb. Technol. 2007, 40, 1788–1793. [Google Scholar] [CrossRef]
- Ren, X.; Buschle-Diller, G. Oxidoreductases for modification of linen fibers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 15–21. [Google Scholar] [CrossRef]
- Tian, L.; Branford-White, C.; Wang, W.; Nie, H.; Zhu, L. Laccase-mediated system pretreatment to enhance the effect of hydrogen peroxide bleaching of cotton fabric. Int. J. Biol. Macromol. 2012, 50, 782–787. [Google Scholar] [CrossRef]
- Pereira, L.; Bastos, C.; Tzanov, T.; Cavaco-Paulo, A.; Guebitz, G.M. Environmentally friendly bleaching of cotton using laccases. Environ. Chem. Lett. 2005, 3, 66–69. [Google Scholar] [CrossRef]
- Neifar, M.; Chouchane, H.; Mahjoubi, M.; Jaouani, A.; Cherif, A. Pseudomonas extremorientalis BU118: A new salt-tolerant laccase-secreting bacterium with biotechnological potential in textile azo dye decolourization. 3 Biotech 2016, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Moya, R.; Hernández, M.; García-Martín, A.B.; Ball, A.S.; Arias, M.E. Contributions to a better comprehension of redox-mediated decolouration and detoxification of azo dyes by a laccase produced by Streptomyces cyaneus CECT 3335. Bioresour. Technol. 2010, 101, 2224–2229. [Google Scholar] [CrossRef]
- Jassal, S.; Warmoota, R.; Sharma, A.; Sheoran, S.; Kumar, D.; Gupta, N. An Economical Industrially Feasible Approach for Denim Biobleaching: Reusing of Laccase and Mediator for Multiple Cycles. Indian J. Microbiol. 2024, 1–13. [Google Scholar] [CrossRef]
- Ryan, S.; Schnitzhofer, W.; Tzanov, T.; Cavaco-Paulo, A.; Gübitz, G.M. An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enzym. Microb. Technol. 2003, 33, 766–774. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Bilal, M.; Li, X.; Shah, S.Z.; Mohamed, B.A.; Hadibarata, T.; Cheng, H. Peroxidases-based enticing biotechnological platforms for biodegradation and biotransformation of emerging contaminants. Chemosphere 2022, 307, 136035. [Google Scholar] [CrossRef]
- Catucci, G.; Valetti, F.; Sadeghi, S.J.; Gilardi, G. Biochemical features of dye-decolorizing peroxidases: Current impact on lignin degradation. Biotechnol. Appl. Biochem. 2020, 67, 751–759. [Google Scholar] [CrossRef]
- Khan, S.; Borah, D. Microbial cell factories in the degradation of azo-dye and their limiting factors: An insight. Clean. Water 2024, 2024, 100034. [Google Scholar] [CrossRef]
- Kumar, V.; Pallavi, P.; Sen, S.K.; Raut, S. Harnessing the potential of white rot fungi and ligninolytic enzymes for efficient textile dye degradation: A comprehensive review. Water Environ. Res. 2024, 96, e10959. [Google Scholar] [CrossRef]
- Ren, J.; Li, X.; Zhang, W.; Li, Z.; Wang, Q.; Li, S.; Wang, S.; Li, H. Evaluation of application potential of dye-decolourizing peroxidase from Bacillus amyloliquefaciens in bioremediation of paper and pulp mill effluent. Front. Microbiol. 2022, 13, 1031853. [Google Scholar] [CrossRef]
- Ilić Đurđić, K.; Ostafe, R.; Prodanović, O.; Đurđević Đelmaš, A.; Popović, N.; Fischer, R.; Schillberg, S.; Prodanović, R. Improved degradation of azo dyes by lignin peroxidase following mutagenesis at two sites near the catalytic pocket and the application of peroxidase-coated yeast cell walls. Front. Environ. Sci. Eng. 2021, 15, 19. [Google Scholar] [CrossRef]
- Saha, P.; Sivaramakrishna, A.; Rao, K.V.B. Bioremediation of reactive orange 16 by industrial effluent-adapted bacterial consortium VITPBC6: Process optimization using response surface methodology (RSM), enzyme kinetics, pathway elucidation, and detoxification. Environ. Sci. Pollut. Res. 2023, 30, 35450–35477. [Google Scholar] [CrossRef]
- Hossain, M.S.; Paul, G.K.; Mahmud, S.; Saleh, M.A.; Uddin, M.S.; Dutta, A.K.; Roy, A.K.; Saha, A.K.; Sheam, M.M.; Ahmed, S.; et al. Mixed dye degradation by Bacillus pseudomycoides and Acinetobacter haemolyticus isolated from industrial effluents: A combined affirmation with wetlab and in silico studies. Arab. J. Chem. 2022, 15, 104078. [Google Scholar] [CrossRef]
- Goswami, D.; Mukherjee, J.; Mondal, C.; Bhunia, B. Bioremediation of azo dye: A review on strategies, toxicity assessment, mechanisms, bottlenecks and prospects. Sci. Total Environ. 2024, 2024, 176426. [Google Scholar] [CrossRef] [PubMed]
- Chahiniana, H.; Sarda, L. Distinction between esterases and lipases: Comparative biochemical properties of sequence-related carboxylesterases. Protein Pept. Lett. 2009, 16, 1149–1161. [Google Scholar] [CrossRef]
- El-Shemy, N.S.; El-Hawary, N.S.; El-Sayed, H. Basic and reactive-dyeable polyester fabrics using lipase enzymes. J. Chem. Eng. Process Technol. 2016, 7, 1000271. [Google Scholar] [CrossRef]
- Kalantzi, S.; Mamma, D.; Kalogeris, E.; Kekos, D. Improved properties of cotton fabrics treated with lipase and its combination with pectinase. Fibres Text. East. Eur. 2010, 18, 86–92. [Google Scholar]
- Khan, M.F.; Kundu, D.; Hazra, C.; Patra, S. A strategic approach of enzyme engineering by attribute ranking and enzyme immobilisation on zinc oxide nanoparticles to attain thermostability in mesophilic Bacillus subtilis lipase for detergent formulation. Int. J. Biol. Macromol. 2019, 136, 66–82. [Google Scholar] [CrossRef]
- Buchert, J.; Pere, J.; Puolakka, A.; Nousiainen, P. Scouring of cotton with pectinases, proteases, and lipases. Text. Chem. Colour. Am. Dyest. Rep. 2000, 32, 48. [Google Scholar]
- Park, Y.J.; Yoon, S.J.; Lee, H.B. A novel thermostable arylesterase from the archaeon Sulfolobus solfataricus P1: Purification, characterization, and expression. J. Bacteriol. 2008, 190, 8086–8095. [Google Scholar] [CrossRef]
- Auterinen, A.L.; Prozzo, B.; Redling, E.; Vermeersch, L.; Yoon, M.Y. Enzymatic Textile Bleaching Compositions and Methods of Use Thereof. U.S. Patent Application 14/220,436, 2014. [Google Scholar]
- Spicka, N.; Tavcer, P.F. New combined bio-scouring and bio-bleaching process of cotton fabrics. Mater. Technol. 2013, 47, 409–412. [Google Scholar]
- Siddiquee, A.B.; Bashar, M.; Sarker, P.; Tohfa, T.T.; Hossan, M.A.; Azad, M.I.; Akhtar, N. Comparative study of conventional and enzymatic pretreatment (scouring and bleaching) of cotton knitted fabrics. Int. J. Eng. Technol. 2014, 3, 37–43. [Google Scholar]
- Kawai, F.; Kawabata, T.; Oda, M. Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustain. Chem. Eng. 2020, 8, 8894–8908. [Google Scholar] [CrossRef]
- Carr, C.M.; Clarke, D.J.; Dobson, A.D. Microbial polyethylene terephthalate hydrolases: Current and future perspectives. Front. Microbiol. 2020, 11, 571265. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B.; Alhussaini, M.S.; Ramteke, P.W. Current applications and future trends of extremozymes in detergent industries. In Microbial Extremozymes; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 223–230. [Google Scholar] [CrossRef]
- Shi, K.; Jing, J.; Song, L.; Su, T.; Wang, Z. Enzymatic hydrolysis of polyester: Degradation of poly (ε-caprolactone) by Candida antarctica lipase and Fusarium solani cutinase. Int. J. Biol. Macromol. 2020, 144, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Carniel, A.; Valoni, É.; Junior, J.N.; da Conceição Gomes, A.; De Castro, A.M. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Process Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature—A new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef]
- Danilova, I.; Sharipova, M. The practical potential of bacilli and their enzymes for industrial production. Front. Microbiol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Demirkan, E.; Kut, D.; Sevgi, T.; Dogan, M.; Baygin, E. Investigation of effects of protease enzyme produced by Bacillus subtilis 168 E6-5 and commercial enzyme on physical properties of woolen fabric. J. Text. Inst. 2020, 111, 26–35. [Google Scholar] [CrossRef]
- El-Sayed, H.; Mowafi, S.; El-Fiky, A.F.; Khalil, E.M. Low temperature water-saving bio-degumming of natural silk using thermophilic protease. Sustain. Chem. Pharm. 2022, 27, 100681. [Google Scholar] [CrossRef]
- Sreelakshmi, S.N.; Vasanthi, N.S.; Saravanan, D. Acidic bacterial proteases from Bacillus species as an alternative agent for scouring of cotton fabrics. J. Text. Inst. 2013, 104, 1118–1124. [Google Scholar] [CrossRef]
- Vigneswaran, C.; Ananthasubramanian, M.; Anbumani, N.; Kandhavadivu, P. Ecofriendly approach to improve pectinolytic reaction and process optimization of bioscouring of organic cotton textiles. J. Eng. Fibers Fabr. 2013, 8, 121–133. [Google Scholar] [CrossRef]
- Pooja, E.S.; Fatima, N. Quality improvement of wool fabric using protease enzyme. Environ. Ecol. Res. 2014, 2, 301–310. [Google Scholar]
- Ndochinwa, O.G.; Wang, Q.Y.; Amadi, O.C.; Nwagu, T.N.; Nnamchi, C.I.; Okeke, E.S.; Moneke, A.N. Current status and emerging frontiers in enzyme engineering: An industrial perspective. Heliyon 2024, 10, e32673. [Google Scholar] [CrossRef]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Nierstrasz, V.A. An overview on biocatalysts immobilisation on textiles: Preparation, progress and application in wastewater treatment. Chemosphere 2021, 279, 130481. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Kundu, D.; Gogoi, M.; Shrestha, A.K.; Karanth, N.G.; Patra, S. Enzyme-responsive and enzyme immobilised nanoplatforms for therapeutic delivery: An overview of research innovations and biomedical applications. Nanopharm. Princ. Appl. 2020, 3, 165–200. [Google Scholar]
- Kakkar, P.; Wadhwa, N. Extremozymes used in textile industry. J. Text. Inst. 2021, 113, 2007–2015. [Google Scholar] [CrossRef]
- Pouresmaeil, M.; Azizi-Dargahlou, S. Factors involved in heterologous expression of proteins in E. coli host. Arch. Microbiol. 2023, 205, 212. [Google Scholar] [CrossRef]
- Vieira Gomes, A.M.; Souza Carmo, T.; Silva Carvalho, L.; Mendonça Bahia, F.; Parachin, N.S. Comparison of yeasts as hosts for recombinant protein production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Nevalainen, H.; Peterson, R. Making recombinant proteins in filamentous fungi—Are we expecting too much? Front. Microbiol. 2014, 5, 75. [Google Scholar]
- Salwoom, L.; Raja Abd. Rahman, R.N.Z.; Salleh, A.B.; Mohd. Shariff, F.; Convey, P.; Mohamad Ali, M.S. New recombinant cold-adapted and organic solvent tolerant lipase from psychrophilic Pseudomonas sp. LSK25, isolated from Signy Island Antarctica. Int. J. Mol. Sci. 2019, 20, 1264. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Sharma, M.; Singh, S.P. Characterization of a novel xylanase from an extreme temperature hot spring metagenome for xylooligosaccharide production. Appl. Microbiol. Biotechnol. 2020, 104, 4889–4901. [Google Scholar] [CrossRef] [PubMed]
- Gil-Durán, C.; Ravanal, M.C.; Ubilla, P.; Vaca, I.; Chávez, R. Heterologous expression, purification and characterization of a highly thermolabile endoxylanase from the Antarctic fungus Cladosporium sp. Fungal Biol. 2018, 122, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.; Walsh, G. Expression and characterisation of a thermophilic endo-1,4-β-glucanase from Sulfolobus shibatae of potential industrial application. Mol. Biol. Rep. 2018, 45, 2201–2211. [Google Scholar] [CrossRef]
- Yu, Z.; Zheng, H.; Zhao, X.; Li, S.; Xu, J.; Song, H. High level extracellular production of a recombinant alkaline catalase in E. coli BL21 under ethanol stress and its application in hydrogen peroxide removal after cotton fabrics bleaching. Bioresour. Technol. 2016, 214, 303–310. [Google Scholar] [CrossRef]
- Chatterjee, A.; Puri, S.; Sharma, P.K.; Deepa, P.R.; Chowdhury, S. Nature-inspired enzyme engineering and sustainable catalysis: Biochemical clues from the world of plants and extremophiles. Front. Bioeng. Biotechnol. 2023, 11, 1229300. [Google Scholar] [CrossRef]
- Rigoldi, F.; Donini, S.; Redaelli, A.; Parisini, E.; Gautieri, A. Engineering of thermostable enzymes for industrial applications. APL Bioeng. 2018, 2, 011501. [Google Scholar] [CrossRef]
- Shrestha, S.; Chio, C.; Khatiwada, J.R.; Mokale Kognou, A.L.; Chen, X.; Qin, W. Optimization of cultural conditions for pectinase production by Streptomyces sp. and characterization of partially purified enzymes. Microb. Physiol. 2023, 33, 12–26. [Google Scholar] [CrossRef]
- Solbak, A.I.; Richardson, T.H.; McCann, R.T.; Kline, K.A.; Bartnek, F.; Tomlinson, G.; Tan, X.; Parra-Gessert, L.; Frey, G.J.; Podar, M.; et al. Discovery of pectin-degrading enzymes and directed evolution of a novel pectate lyase for processing cotton fabric. J. Biol. Chem. 2005, 280, 9431–9438. [Google Scholar] [CrossRef]
- Li, P.; Wei, X.; Wang, Y.; Liu, H.; Xu, Y.; Zhang, Z.; Li, J.; Wang, J.; Guo, C.; Sui, S.; et al. Improvement of optimum pH and specific activity of pectate lyase from Bacillus RN. 1 using loop replacement. Front. Bioeng. Biotechnol. 2023, 11, 1242123. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilisation of enzymes and surface analysis techniques for immobilised enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Khan, M.F.; Gogoi, M.; Patra, S. Environmental impact and econanotoxicity of engineered nanomaterials. In Nanotoxicology and Nanoecotoxicology; Kumar, V., Guleria, P., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 1, pp. 287–312. [Google Scholar]
- Khan, M.F.; Murphy, C.D. Environmental remediation by novel nanomaterials and fungi with high-degradation capacity of hazardous contaminants. In Bio and Nanoremediation of Hazardous Environmental Pollutants; Fernández-Luqueño, F., López-Valdez, F., Medina-Pérez, G., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 283–310. [Google Scholar]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties—Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef]
- Costa, S.A.; Tzanov, T.; Paar, A.; Gudelj, M.; Gübitz, G.M.; Cavaco-Paulo, A. Immobilization of catalases from Bacillus SF on alumina for the treatment of textile bleaching effluents. Enzym. Microb. Technol. 2001, 28, 815–819. [Google Scholar] [CrossRef]
- Costa, S.A.; Tzanov, T.; Carneiro, F.; Gübitz, G.M.; Cavaco-Paulo, A. Recycling of textile bleaching effluents for dyeing using immobilized catalase. Biotechnol. Lett. 2002, 24, 173–176. [Google Scholar] [CrossRef]
- Narayanan, M.P.; Murugan, S.; Eva, A.S.; Devina, S.U.; Kalidass, S. Application of immobilized laccase from Bacillus subtilis MTCC 2414 on decolourization of synthetic dyes. Res. J. Microbiol. 2015, 10, 421–432. [Google Scholar]
- Sankarraj, N.; Nallathambi, G. Enzymatic biopolishing of cotton fabric with free/immobilized cellulase. Carbohydr. Polym. 2018, 191, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Meenakshisundaram, S.; Selvakumar, N. Conservation of cellulase enzyme in biopolishing application of cotton fabrics. J. Text. Inst. 2008, 99, 339–346. [Google Scholar] [CrossRef]
- Dinçer, A.; Telefoncu, A. Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. J. Mol. Catal. B Enzym. 2007, 45, 10–14. [Google Scholar] [CrossRef]
- Antecka, K.; Zdarta, J.; Siwińska-Stefańska, K.; Sztuk, G.; Jankowska, E.; Oleskowicz-Popiel, P.; Jesionowski, T. Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts 2018, 8, 402. [Google Scholar] [CrossRef]
- de Souza Lima, J.; Immich, A.P.S.; de Araújo, P.H.H.; de Oliveira, D. Cellulase immobilized on kaolin as a potential approach to improve the quality of knitted fabric. Bioprocess Bioproc. Eng. 2022, 45, 679–688. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Silvério, S.C.; Tavares, A.P.; Brígida, A.I.S.; Loureiro, J.M.; Boaventura, R.A.; Macedo, E.A.; Coelho, M.A.Z. Green coconut fiber: A novel carrier for the immobilization of commercial laccase by covalent attachment for textile dyes decolourization. World J. Microbiol. Biotechnol. 2012, 28, 2827–2838. [Google Scholar] [CrossRef]
- Sondhi, S.; Sharma, P.; Saini, S.; Puri, N.; Gupta, N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS ONE 2014, 9, e96951. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, S.M.; Tahoon, M.A.; Mnif, W.; Ben Rebah, F. Iron oxide/chitosan magnetic nanocomposite immobilized manganese peroxidase for decolourization of textile wastewater. Processes 2019, 8, 5. [Google Scholar] [CrossRef]
- Silva, C.J.; Gübitz, G.; Cavaco-Paulo, A. Optimisation of a serine protease coupling to Eudragit S-100 by experimental design techniques. J. Chem. Technol. Biotechnol. 2006, 81, 8–16. [Google Scholar] [CrossRef]
- Srivastava, B.; Singh, H.; Khatri, M.; Singh, G.; Arya, S.K. Immobilization of keratinase on chitosan grafted-β-cyclodextrin for the improvement of the enzyme properties and application of free keratinase in the textile industry. Int. J. Biol. Macromol. 2020, 165, 1099–1110. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Gursel, I.; Yilmaz, M.; Arica, M.Y. Immobilization of laccase on itaconic acid grafted and Cu (II) ion chelated chitosan membrane for bioremediation of hazardous materials. J. Chem. Technol. Biotechnol. 2012, 87, 530–539. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, M.; Hu, H.; Zhang, X. Chitosan beads immobilized manganese peroxidase catalytic potential for detoxification and decolourization of textile effluent. Int. J. Biol. Macromol. 2016, 89, 181–189. [Google Scholar] [CrossRef]
- Sondhi, S.; Kaur, R.; Kaur, S.; Kaur, P.S. Immobilization of laccase-ABTS system for the development of a continuous flow packed bed bioreactor for decolourization of textile effluent. Int. J. Biol. Macromol. 2018, 117, 1093–1100. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, J.; Wang, Q.; Fan, X.; Wang, P.; Sun, X. Immobilization of cellulases on the reversibly soluble polymer Eudragit S-100 for cotton treatment. Eng. Life Sci. 2013, 13, 194–200. [Google Scholar] [CrossRef]
- Chakravorty, D.; Khan, M.F.; Patra, S. Multifactorial level of extremostability of proteins: Can they be exploited for protein engineering? Extremophiles 2017, 21, 419–444. [Google Scholar] [CrossRef]
- El-Sayed, H.; El-Fiky, A.F.; Mowafi, S. Extremozymes as future appropriate benign elements for eco-friendly wet processing of wool and silk. J. Nat. Fibers 2022, 19, 15035–15044. [Google Scholar] [CrossRef]
- Kiadehi, M.S.H.; Amoozegar, M.A.; Asad, S.; Siroosi, M. Exploring the potential of halophilic archaea for the decolourization of azo dyes. Water Sci. Technol. 2018, 77, 1602–1611. [Google Scholar] [CrossRef]
- Kumari, A.; Kishor, N.; Guptasarma, P. Characterization of a mildly alkalophilic and thermostable recombinant Thermus thermophilus laccase with applications in decolourization of dyes. Biotechnol. Lett. 2018, 40, 285–295. [Google Scholar] [CrossRef]
- Saravanan, D.; Prakash, A.A.; Jagadeeshwaran, D.; Nalankilli, G.; Ramachandran, T.; Prabakaran, C. Optimization of thermophile Bacillus licheniformis -amylase desizing of cotton fabrics. Indian J. Fibre Text. Res. 2011, 36, 253–258. [Google Scholar]
- Aygan, A.; Arikan, B.; Korkmaz, H.; Dinçer, S.; Çolak, Ö. Highly thermostable and alkaline α-amylase from a halotolerant-alkaliphilic Bacillus sp. AB68. Braz. J. Microbiol. 2008, 39, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Anish, R.; Rahman, M.S.; Rao, M. Application of cellulases from an alkalothermophilic Thermonospora sp. in biopolishing of denims. Biotechnol. Bioeng. 2007, 96, 48–56. [Google Scholar] [CrossRef]
- Wiedemann, S.G.; Clarke, S.J.; Nguyen, Q.V.; Cheah, Z.X.; Simmons, A.T. Strategies to reduce environmental impacts from textiles: Extending clothing wear life compared to fibre displacement assessed using consequential LCA. Resour. Conserv. Recycl. 2023, 198, 107119. [Google Scholar] [CrossRef]
- Toprak, T.; Anis, P. Textile industry’s environmental effects and approaching cleaner production and sustainability, an overview. J. Text. Eng. Fash. Technol. 2017, 2, 429–442. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-sustainability of the textile production: Waste recovery and current recycling in the composites world. Polymers 2020, 13, 134. [Google Scholar] [CrossRef]
- Frondel, M.; Horbach, J.; Rennings, K. End-of-pipe or cleaner production? An empirical comparison of environmental innovation decisions across OECD countries. Bus. Strategy Environ. 2007, 16, 571–584. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, S.J.; Rose, N.M. Eco–textiles: For sustainable development. Int. J. Sci. Eng. Res. 2013, 4, 1379–1390. [Google Scholar]
- Panda, S.K.B.C.; Sen, K.; Mukhopadhyay, S. Sustainable pretreatments in textile wet processing. J. Clean. Prod. 2021, 329, 129725. [Google Scholar] [CrossRef]
- Islam, S.; Jalil, M.A.; Belowar, S.; Saeed, M.A.; Hossain, S.; Rahamatolla, M.; Ali, S. Role of mordants in natural fabric dyeing and their environmental impacts. Environ. Sci. Pollut. Res. 2024, 32, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Pranta, A.D.; Rahaman, M.T. Extraction of eco-friendly natural dyes and biomordants for textile colorations: A critical review. Nano Struct. Nano Objects 2024, 39, 101243. [Google Scholar] [CrossRef]
- Asmelash, F.; Ayele, M. Beneficiation of Commiphora africana plant: Extraction and application of green softener on cotton fabric. J. Eng. 2021, 2021, 9910707. [Google Scholar] [CrossRef]
- Szostak-Kotowa, J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Egan, J.; Salmon, S. Strategies and progress in synthetic textile fiber biodegradability. SN Appl. Sci. 2022, 4, 22. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M. Strategies of recovery and organic recycling used in textile waste management. Int. J. Environ. Res. Public Health 2022, 19, 5859. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef]
- Suen, D.W.S.; Chan, E.M.H.; Lau, Y.Y.; Lee, R.H.P.; Tsang, P.W.K.; Ouyang, S.; Tsang, C.W. Sustainable textile raw materials: Review on bioprocessing of textile waste via electrospinning. Sustainability 2023, 15, 11638. [Google Scholar] [CrossRef]
- Liu, X.; Sathishkumar, K.; Zhang, H.; Saxena, K.K.; Zhang, F.; Naraginiti, S.; Anbarasu, K.; Rajendiran, R.; Aruliah, R.; Guo, X. Frontiers in environmental cleanup: Recent advances in remediation of emerging pollutants from soil and water. J. Hazard. Mater. Adv. 2024, 16, 100461. [Google Scholar] [CrossRef]
- Kabir, S.M.M.; Koh, J. Sustainable textile processing by enzyme applications. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, K.F., de Sousa, R.N., Mielke, K.C., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Introduction to enzymes. In Sustainable Technologies for Fashion and Textiles; Woodhead Publishing: Cambridge, UK, 2020; pp. 75–90. [Google Scholar]
- Vigneswaran, C.; Ananthasubramanian, M.; Kandhavadivu, P. Bioprocessing of Textiles; Woodhead Publishing India Pvt Limited: New Delhi, India, 2014. [Google Scholar]
- Fasim, A.; More, V.S.; More, S.S. Large-scale production of enzymes for biotechnology uses. Curr. Opin. Biotechnol. 2021, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.S.G.; Tominaga, F.K.; Rosa, J.M.; Borrely, S.I. Emerging pollutants in textile wastewater: An ecotoxicological assessment focusing on surfactants. Environ. Sci. Pollut. Res. 2024, 31, 27817–27828. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1. [Google Scholar] [CrossRef]
| Fabric Type | Example | Dye type Used for Dyeing * |
|---|---|---|
| Synthetic fibres | ||
| Polyester | Dacron, Terylene | Disperse, pigment |
| Polyamide | Nylon, Perlon, Rilsan | Acid, reactive, disperse, mordant, pigment |
| Polyacrylonitrile | Acrilan, Courtelle, Orlon | Basic, disperse, pigment |
| Polyolefines | Meraklon, Prolene | Disperse |
| Polyvinyl chloride | Envilon, Thermovyl | Basic, disperse |
| Elastomers | Glospan, Lycra | Acid, disperse, reactive (wool), vat |
| Natural fibres | ||
| Silk | - | Azoic, basic, direct, oxidation, reactive, mordant, sulphur, vat |
| Wool and wool blends | Wool–cotton, wool–viscose, etc. | Acid, basic, reactive, mordant, solubilised vat |
| Cotton | - | Azoic, basic, direct, oxidation, reactive, mordant, sulphur, vat |
| Modified cellulose fibres | Viscose, secondary acetate, triacetate | Disperse, direct, pigment, reactive, mordant, sulphur, vat, solubilised vat |
| Bast | Linen, flax, ramie, hemp, jute | Acid, direct, reactive, disperse, vat, solubilised vat |
| Textile Process | Toxic Chemicals | CAS Number | Hazardous Causes |
|---|---|---|---|
| Yarn sizing | Polyvinyl alcohol | 9002-89-5 | Eye irritation; discomfort in inhalation |
| Size preservative | Pentachlorophenol | 87-86-5 | Eye irritation; adverse neurological, blood, and liver effects |
| Formaldehyde | 50-00-0 | Skin and eye irritation; coughing; wheezing; nausea; burning sensations in the nose and throat | |
| Coating and degreasing | Synthetic non-biodegradable surfactants + solvents | - | Dermatological compatibility, toxicity, and biodegradability |
| Anti-caking agent in salt | Cyanide | 57-12-5 | Eyes, nose, and throat irritation; headache; prolonged exposure can cause coma and even death |
| Biocide on hosiery and fabrics | Tributyltin oxide | 56-35-9 | Extremely hazardous to aquatic lives |
| Bleaching | Calcium hypochlorite | 7778-54-3 | Coughing and breath shortness |
| Sodium hypochlorite | 7681-52-9 | Skin irritation; coughing; stomach and abdominal pain | |
| Peroxide stabiliser | Sodium silicate | 1344-09-8 | Skin and eye irritation |
| Phosphorous-based compounds (Tris(2,4-di-tert-butylphenyl)phosphite) | 31570-04-4 | Causing explosion hazards | |
| Detergents and emulsifiers | Non-ionic surfactants (octylphenol ethoxylate) | 9036-19-5 | Skin irritation; environmental risks |
| Nonylphenol–ethylene oxide adducts (APEOs) | 26027-38-3 | Extremely toxic to aquatic organisms | |
| Stain removers | Carbon tetrachloride | 56-23-5 | Harmful effect on the liver, central nervous system, and kidneys; extended exposure can cause coma and fatality |
| Softener | Silicones and amino silicones with APEO emulsifier | 63148-62-0; 63148-62-9 | Chronic respiratory effects; extremely toxic to aquatic organisms |
| Dyes | Azo dyes (Remazol Brilliant Blue R) | 12222-67-8 | Carcinogenic, skin irritation, aquatic toxicity, persistent in ecosystems |
| Reactive dyes (Procion blue) | 12236-36-4 | Skin irritation, allergic reactions, aquatic toxicity | |
| Acid dyes (Cibacron fuchsia) | 6105-59-9 | Skin irritation, heavy metal contamination, water pollution | |
| Basic dyes (crystal violet) | 548-62-9 | Dermatitis, respiratory irritation, aquatic toxicity | |
| Disperse dyes (Disperse Blue 1) | 12222-72-5 | Allergic dermatitis, suspected carcinogen, water pollution | |
| Sulphur dyes (sulphur black) | 1326-97-6 | Skin irritation, respiratory issues, aquatic toxicity | |
| Vat dyes (indigo) | 482-89-3 | Respiratory irritation, water toxicity, persistent pollutants | |
| Pigments (titanium dioxide) | 13463-67-7 | Heavy metal toxicity, carcinogenic, non-biodegradable | |
| Heavy metals | Copper (Cu) | 7440-50-8 | Inhalation and respiratory problems; fever; nausea; vomiting |
| Lead (Pb) | 7439-92-1 | Affects the central nervous system, causing coma, convulsions, and death | |
| Mercury (Hg) | 7439-97-6 | Adverse effects on the nervous, digestive, and immune systems | |
| Cadmium (Cd) | 7440-43-9 | Harmful to lungs, bones, and kidneys | |
| Chromium (Cr) | 7440-47-3 | Pulmonary sensitisation; causes lung, nasal, and sinus cancer; severe dermatitis and painless skin ulcers | |
| Arsenic (As) | 7440-38-2 | Affects organs such as the eyes, skin, liver, kidneys, and lungs; causes cancer | |
| Carriers in dyeing | Dichlorobenzene | 25321-22-6 | Kidney and liver cancer |
| Trichlorobenzene | 12002-48-1 | Skin, eye, nose, and throat irritation | |
| Oxidation in dyeing | Sodium dichromate | 10588-01-9 | Causes asthma; damages the liver and kidneys |
| Pigment printing and dye-fixing | Kerosene | 8008-20-6 | Dizziness, headache, and vomiting |
| Formaldehyde | 50-00-0 | Skin and eye irritation; coughing; wheezing; nausea; burning sensations in the nose and throat | |
| Finishing | Cationic surfactants (cetyltrimethylammonium bromide) | 57-09-0 | Skin irritation |
| Functional synthetic finish (perfluorooctanoic acid) | 335-67-1 | Environmental risks; toxic to aquatic organisms |
| Textile Effluent Remediation | Fungi | Bacteria | Algae |
|---|---|---|---|
| Efficiency | - High ability to degrade a wide range of dyes - Effective in removing heavy metals such as chromium and copper - High tolerance to toxic compounds | - Efficient in decolourising dyes and removing textile pollutants - Faster growth rate compared to fungi - Can be engineered for enhanced degradation | - Effective in removing dye pollutants through biosorption and bioaccumulation - Can absorb heavy metals from effluent |
| Advantages | - High degradation potential - Efficient in breaking down complex dyes and pollutants - Can be used in both solid-state and liquid-state remediation | - Fast growth rate and ability to adapt to changing conditions - Can be used in both aerobic and anaerobic environments - Lower nutrient requirements | - Easy to cultivate in large quantities - Can be used in combination with other microorganisms - Cost-effective |
| Limitations | - Slow growth rate - Requires specific conditions - May need additional treatment to handle residual metabolites | - Sensitive to toxic concentrations of pollutants - May require genetic modification for enhanced degradation | - Limited to certain types of dyes - Requires light for photosynthesis - Growth is sensitive to environmental changes |
| Applications | - Bioremediation of textile wastewater - Removal of toxic dyes and heavy metals from effluents | - Biodegradation of textile dyes and chemicals - Treatment of wastewater with high concentrations of pollutants | - Bioremediation of dyes and heavy metals - Integrated treatment systems with fungi and bacteria |
| Reference | [21,23] | [24,25] | [24,26] |
| Bacterial Species | Dye | % Degradation | Degradation Conditions (Dye conc., pH, Temp., Inc. Time) | Enzyme Involved | Reference |
|---|---|---|---|---|---|
| Isolated single culture | |||||
| Actinomycetes strains | Orange dye | 85 | 50 mg/L, pH 7.2, 37 °C, 48 h | Not identified | [41] |
| Aeromonas hydrophila | Crystal violet | 99 | 100 mg/L, pH 7, 35 °C, 8 h | Laccase, lignin peroxidase | [42] |
| Alcaligenes sp. TEX S6 | Direct Red 28 | 86 | 150 mg/L, pH 7, 37 °C, 48 h | Not identified | [43] |
| Bacillus lentus BI377 | Reactive Red 141, Reactive Red 2 | 99.11 | 500 mg/L, pH 8, 40 °C, 6 h | Superoxide dismutase, peroxidase | [44] |
| Bacillus megaterium KY848339 | Acid Red 337 | 91 | 500 mg/L, pH 7, 30 °C, 24 h | Azoreductase | [45] |
| Bacillus sp. | Direct Red 81 | - | 100 mg/L, pH 7, 30 °C, 24 h | Azoreductase, laccase | [46] |
| Klebsiella pneumoniae | Methyl orange | 83 | 20 μM, pH 8, 40 °C, 10 min | Azoreductase | [47] |
| Marinobacter sp. HBRA | Direct Blue 1 | 100 | 100 mg/L, pH 8, 37 °C, 6 h | Oxidases (not identified) | [48] |
| Shewanella sp. | Reactive Black-5, Direct Red-81, Acid Red-88 | 96.9 | 200 mg/L, pH 8.5, 35 °C, 12 h | Azoreductase | [49] |
| Bacillus sp. strain CICC 23870 | Methyl orange, Black 5, Acid Blue 113, methyl red | 97.87 | 32.7 mg/L, pH 7, 35 °C, 24 h | Azoreductase | [50] |
| Mixed culture | |||||
| Brevibacillus aydinogluensis, Geobacillus thermoleovorans, Anoxybacillus flavithermus, Bacillus thermoamylovorans | Direct Black G, Direct Black 38, Congo red, methyl orange | 97 | 600 mg/L, pH 8, 55 °C, 8 h | Azoreductase, laccase, lignin peroxidase, manganese peroxidase | [51] |
| Enterococcus sp. L2 and Mycobacterium vaccae | Reactive Violet 5R | 97.6 | 100 mg/L, pH 6.8, 8 h | Azoreductase, formate dehydrogenase | [52] |
| Lysinibacillus sp., Raoultella sp., Enterococcus spp., Citrobacter sp., Lysinibacillus sp. | Reactive Black 1 | 99 | 100 mg/L, pH 7, 37 °C, 8 h | Azoreductase, lignin peroxidase | [53] |
| Textile Process | Existing Harmful Chemicals | Green Chemical Substitute | Enzymatic Alternative | Reference |
|---|---|---|---|---|
| Sizing | Polyvinyl alcohol | Potato starch; carboxymethylcellulose | - | [60] |
| Desizing | Mineral acids (acid-based desizing) | - | Amylase; xylanase | [12] |
| Scouring | Sodium hydroxide (caustic soda) | - | Pectinase (pectin lyase); xylanase | [61] |
| Stonewashing and polishing | Nonylphenol–ethylene oxide adducts [alkylphenol polyethoxylates (APEOs)] | Fatty alcohol–ethylene oxide adducts; alkyl polyglycosides | Cellulase | [62] |
| Bleaching | Calcium and sodium hypochlorite; other chlorine oxidising chemicals | Hydrogen peroxide; ozone at cold | Glucose-oxidase; laccase; ligninase (lignin peroxidase); arylesterase | [63,64] |
| Bleach clean-up | Thiosulfates | - | Catalase | [65] |
| Dyeing and printing | Kerosene; formaldehyde; dichlorobenzene; trichlorobenzene | Water-based thickener; polycarboxylic acids; non-formaldehyde products; butyl benzoate; benzoic acid | Ligninase (lignin peroxidase) | [5] |
| Finishing and effluent treatment | Silicone-based softeners; formaldehyde-based resins; heavy metals | Natural oils (e.g., soybean, castor, or palm oils); formaldehyde-free resins; eco-friendly coagulants | Proteases; lipases; laccase | [3,64] |
| Immobilisation Method | Enzyme | Carrier/Support | Improvement | Stability/Reusability | Reference |
|---|---|---|---|---|---|
| Adsorption | Cellulase | Ca alginate starch bead | Lower weight loss, minimal tensile strength reduction (67–98.35%), and improved whiteness index | - | [181] |
| Cellulase | Epoxy resin | Maximum activity was used on cotton fabric for biopolishing | Reuse up to 6 cycles | [182] | |
| Cellulase | PVA coated chitosan | pH optimum of enzyme shifted from 4.0 to 7.0 and showed better stability at neutral pH | Stability of 52% after 8 cycles | [183] | |
| Laccase | TiO2–ZrO2–SiO2 | 100% alizarin red S removal | 90% stable after 20 days | [184] | |
| Adsorption and covalent bonding | Cellulase | Kaolin | Minimised tensile strength loss, enzyme reusability, improved product quality, and reduced polishing costs | Better recovery and reuse for 3 cycles | [185] |
| Covalent binding | Laccase | Green coconut fibre | Enhanced thermal stability at 50 °C and high efficiency in continuous reactive dye decolourisation | Stability retained 45–50% after 4 cycles | [186] |
| Laccase | Glycidyl methacrylate functionalised polyacrylamide alginate | 55% dye removal | 50% stability remained after 5 cycles | [187] | |
| Manganese peroxidase | Fe3O4/chitosan | 98% Reactive Orange 16 and 96% methylene blue removal | 86% stable after 5 cycles and 60% after 14 days | [188] | |
| Covalent/cross-linking | Protease (esperase) | Eudragit S-100 | Operational stability at 60 °C improved 1.7-fold | Activity of 72% after 5 cycles | [189] |
| Cross-linking | Keratinase | Chitosan-β-cyclodextrin | Enhanced optimal activity at pH 11 and 70–75 °C with superior thermo-stability | Storage stability of ~53% after 30 days | [190] |
| Laccase | Cu(II) ion chelated chitosan | 43% removal of methyl orange, 69% removal of Cibacron blue and 87% removal of Reactive Black 5 | 81% stable after 20 uses | [191] | |
| Manganese peroxidase | Chitosan beads | 97% dye removal | 60% stable after 10 cycles | [192] | |
| Entrapment | Laccase | Alginate beads | 66% dye removal | 95% stable after 15 days | [193] |
| Non-covalent binding | Cellulase | Eudragit S-100 and Eudragit L-100 | Improved fabric softness while minimising weight and tensile strength loss to the surface fibres | Stability of 51% and 42% after 3 cycles | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.F. Recent Advances in Microbial Enzyme Applications for Sustainable Textile Processing and Waste Management. Sci 2025, 7, 46. https://doi.org/10.3390/sci7020046
Khan MF. Recent Advances in Microbial Enzyme Applications for Sustainable Textile Processing and Waste Management. Sci. 2025; 7(2):46. https://doi.org/10.3390/sci7020046
Chicago/Turabian StyleKhan, Mohd Faheem. 2025. "Recent Advances in Microbial Enzyme Applications for Sustainable Textile Processing and Waste Management" Sci 7, no. 2: 46. https://doi.org/10.3390/sci7020046
APA StyleKhan, M. F. (2025). Recent Advances in Microbial Enzyme Applications for Sustainable Textile Processing and Waste Management. Sci, 7(2), 46. https://doi.org/10.3390/sci7020046






