Additional Health Benefits Observed following a Nature Walk Compared to a Green Urban Walk in Healthy Females
Abstract
1. Introduction
1.1. Beneficial Effects of Physical Activity
1.2. Beneficial Effects of Exposure to Nature
1.3. The Current Study
2. Materials and Methods
2.1. Participants
2.2. Intervention Protocol
2.3. Measurements
2.3.1. Mood
2.3.2. Physiological Stress
2.3.3. Energy Intake and Ghrelin
2.3.4. Other Measures
2.4. Statistical Analysis
3. Results
3.1. Participants
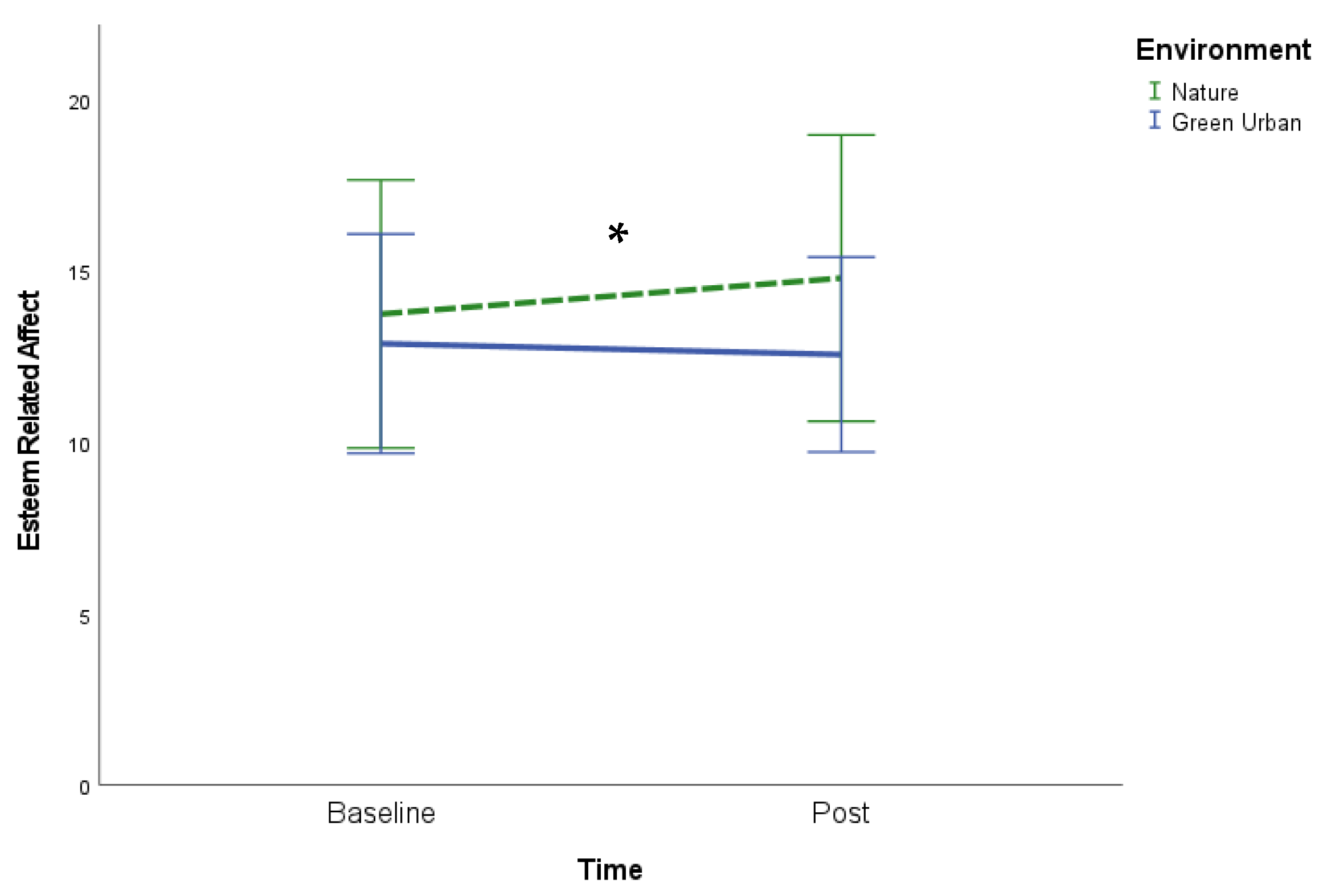
3.2. Mood
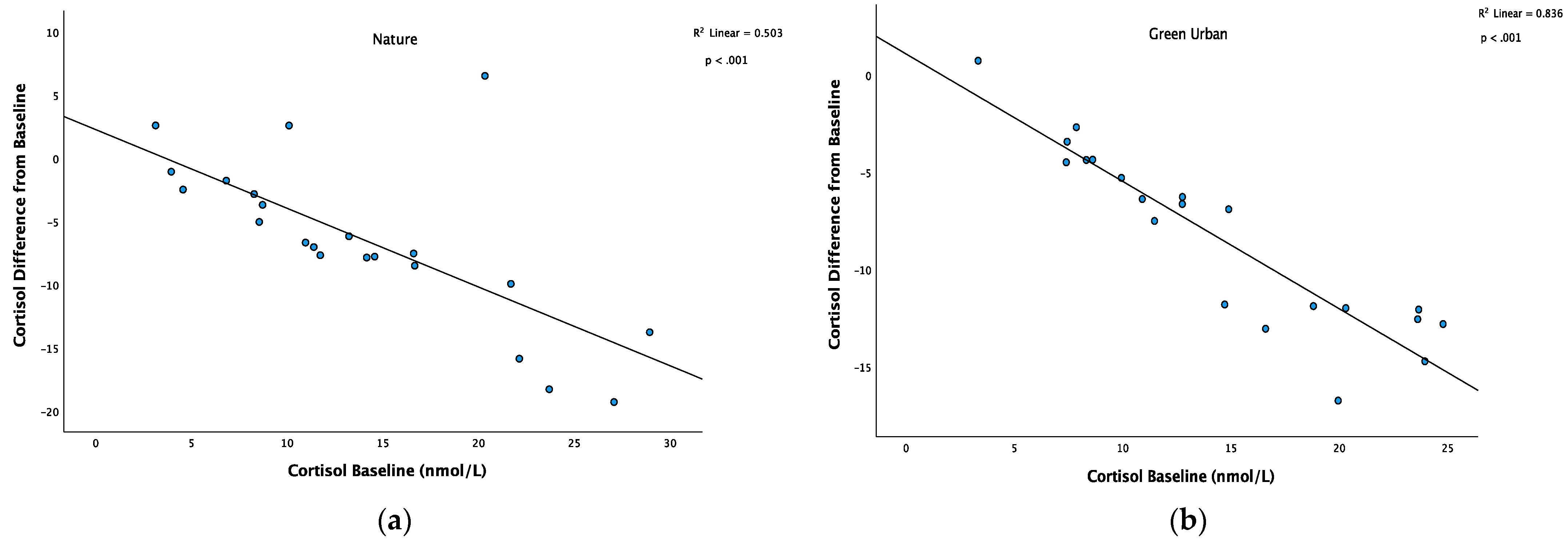
3.3. Physiological Stress
3.4. Energy Intake and Ghrelin
4. Discussion
4.1. Benefits of Walking Outdoors
4.2. Additional Benefits of Walking in Nature
4.3. Eating Behaviour
4.4. Limitation
5. Conclusions & Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fink, G. Stress: The Health Epidemic of the 21st Century; Elsevier SciTech Connect: Cambridge, MA, USA, 2016; Available online: https://scitechconnect.elsevier.com/stress-health-epidemic-21st-century/#:~:text=%E2%80%9CStress%E2%80%9D%20has%20been%20dubbed%20the,physical%20health%20can%20be%20devastating (accessed on 19 May 2023).
- Stress: Are We Coping? Mental Health Foundation: London, UK, 2018.
- Chrousos, G. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L.; et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef]
- Fisher, J.; Young, C.; Fadel, P. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. Basic Clin. 2009, 148, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Stults-Kolehmainen, M.; Bartholomew, J. Psychological stress impairs short-term muscular recovery from resistance exercise. Med. Sci. Sport. Exerc. 2012, 44, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef]
- Goldstein, D.S. Stress-induced activation of the sympathetic nervous system. Baillieres. Clin. Endocrinol. Metab. 1987, 1, 253–278. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Kirschbaum, C. Sex differences in HPA axis responses to stress: A review. Biol. Psychol. 2005, 69, 113–132. [Google Scholar] [CrossRef]
- Thau, L.; Gandhi, J.; Sharma, S. Physiology, Cortisol; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://europepmc.org/article/NBK/NBK538239 (accessed on 15 February 2023).
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Stress Effects on the Body. American Psychological Association. 2023. Available online: https://www.apa.org/topics/stress/body (accessed on 27 July 2023).
- NHS. Obesity Overview. 2023. Available online: https://www.nhs.uk/conditions/obesity/ (accessed on 24 February 2023).
- Tomiyama, A.J. Stress and Obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Obesity and Overweight. 2023. Available online: https://www.cdc.gov/nchs/fastats/obesity-overweight.htm (accessed on 15 May 2023).
- Office of Health Improvements & Disparities. Obesity Profile. 2023. Available online: https://www.gov.uk/government/statistics/obesity-profile-update-may-2023 (accessed on 15 May 2023).
- Salmon, P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar] [CrossRef]
- Singh, B.; Olds, T.; Curtis, R.; Dumuid, D.; Virgara, R.; Watson, A.; Szeto, K.; O’Connor, E.; Ferguson, T.; Eglitis, E.; et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: An overview of systematic reviews. Br. J. Sports Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Whitney Nicol, C.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Fam. Med. Prim. Care Rev. 2006, 8, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- UK Chief Medical Officers’ Physical Activity Guidelines. 2019. Available online: https://www.gov.uk/government/publications/physical-activity-guidelines-uk-chief-medical-officers-report (accessed on 23 June 2023).
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.; Morton, J. Benefits of weight loss of 10% or more in patients with overweight or obesity: A review. Obesity 2022, 30, 802–840. [Google Scholar] [CrossRef]
- Laskowski, E.R. The role of exercise in the treatment of obesity. PM R 2012, 4, 840–844. [Google Scholar] [CrossRef]
- Conger, S.A.; Toth, L.P.; Cretsinger, C.; Raustorp, A.; Mitáš, J.; Inoue, S.; Bassett, D.R. Time Trends in Physical Activity Using Wearable Devices: A Systematic Review and Meta-analysis of Studies from 1995 to 2017. Med. Sci. Sports Exerc. 2022, 54, 288–298. [Google Scholar] [CrossRef]
- NHS. Statistics on Obesity, Physical Activity and Diet, England, 2019. 2019. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/statistics-on-obesity-physical-activity-and-diet-england-2019/part-5-adult-physical-activity#adult-physical-activity (accessed on 16 February 2022).
- WHO. Physical Activity. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 18 February 2023).
- Mavrantza, A.M.; Bigliassi, M.; Calogiuri, G. Psychophysiological mechanisms underlying the effects of outdoor green and virtual green exercise during self-paced walking. Int. J. Psychophysiol. 2022, 184, 39–50. [Google Scholar] [CrossRef]
- Hartig, T. Restoration in nature: Beyond the conventional narrative. Nat. Psychol. Biol. Cogn. Dev. Soc. Pathw. Well-Being 2021, 67, 89–151. [Google Scholar]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef]
- Hansen, M.; Jones, R.; Tocchini, K. Shinrin-yoku (Forest bathing) and nature therapy: A state-of-the-art review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, H.; Bratman, G.N.; Breslow, S.J.; Cochran, B.; Kahn, P.H., Jr.; Lawler, J.J.; Levin, P.S.; Tandon, P.S.; Varanasi, U.; Wolf, K.L.; et al. Nature contact and human health: A research agenda. Environ. Health Perspect. 2017, 125, 075001. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Donelli, D.; Carlone, L.; Maggini, V.; Firenzuoli, F.; Bedeschi, E. Effects of forest bathing (shinrin-yoku) on individual well-being: An umbrella review. Int. J. Environ. Health Res. 2022, 32, 1842–1867. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, M.; Wood, C.; Pretty, J.; Schoenmakers, P.; Bloomfield, D.; Barton, J. Regular Doses of Nature: The Efficacy of Green Exercise Interventions for Mental Wellbeing. Int. J. Environ. Res. Public Health 2020, 17, 1526. [Google Scholar] [CrossRef]
- Lachowycz, K.; Jones, A.P. Greenspace and obesity: A systematic review of the evidence. Obes. Rev. 2011, 12, 183–189. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, W.; Liu, X.; Markevych, I.; Bloom, M.S.; Zhao, T.; Heinrich, J.; Yang, B.; Dong, G. Greenspace with overweight and obesity: A systematic review and meta-analysis of epidemiological studies up to 2020. Obes. Rev. 2020, 21, e13078. [Google Scholar] [CrossRef]
- Sillman, D.; Rigolon, A.; Browning, M.; Yoon, H.; McAnirlin, O. Do sex and gender modify the association between green space and physical health? A systematic review Environ. Res. 2022, 209, 112869. [Google Scholar] [CrossRef]
- Biswas, R. Urban Eco-Psychological Attitude during COVID 19 ‘Lockdown’: A Survey. Int. J. Creat. Res. Thoughts 2020, 8, 3017–3037. [Google Scholar]
- Alcock, I.; White, M.P.; Wheeler, B.W.; Fleming, L.E.; Depledge, M.H. Longitudinal effects on mental health of moving to greener and less green urban areas. Environ. Sci. Technol. 2014, 48, 1247–1255. [Google Scholar] [CrossRef]
- Pretty, J.; Peacock, J.; Sellens, M.; Griffin, M. The mental and physical health outcomes of green exercise. Int. J. Environ. Health Res. 2005, 15, 319–337. [Google Scholar] [CrossRef]
- Li, Q. Effects of forest environment (Shinrin-yoku/Forest bathing) on health promotion and disease prevention—The Establishment of ‘Forest Medicine’. Environ. Health Prev. Med. 2022, 27, 43. [Google Scholar] [CrossRef]
- Barton, J.; Bragg, R.; Wood, C.; Pretty, J. Green Exercise: Linking Nature, Health and Well-Being; Routledge: Abingdon, UK, 2016. [Google Scholar]
- Gladwell, V.; Brown, D.; Wood, C.; Sandercock, G.; Barton, J. The great outdoors: How a green exercise environment can benefit all. Extrem. Physiol. Med. 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Lahart, I.; Darcy, P.; Gidlow, C.; Calogiuri, G. The effects of green exercise on physical and mental wellbeing: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 1352. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.X.; Lan, X.G.; Cao, Y.B.; Chen, Z.M.; He, Z.H.; Lv, Y.D.; Wang, Y.Z.; Hu, X.L.; Wang, G.F. Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province, China. Biomed. Environ. Sci. 2012, 25, 317–324. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)—Using salivary cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef]
- Toda, M.; Den, R.; Hasegawa-Ohira, M.; Morimoto, K. Effects of woodland walking on salivary stress markers cortisol and chromogranin A. Complement. Ther. Med. 2013, 21, 29–34. [Google Scholar] [CrossRef]
- Tsunetsugu, Y.; Park, B.J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) in an old-growth broadleaf forest in Yamagata Prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar]
- Cohen, J. Statistcal Power Analysis for the Behaviousal Sciences; Second Edi.6; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988; Volume 6. [Google Scholar]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by α-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Buffenstein, R.; Poppitt, S.D.; McDevitt, R.M.; Prentice, A.M. Food Intake and the Menstral cycle: A Retrospective Analysis, With Implications for Appetite Research. Physiol. Behav. 1995, 58, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Grove, R.; Prapavessis, H. Abbreviated POMS Questionnaire (Items and Scoring Key). No. April. 1993. Available online: https://www.researchgate.net/publication/258239009_Abbreviated_POMS_Questionnaire_40_items (accessed on 27 July 2023).
- Aydin, S.; Halifeoglu, İ.; Ozercan, İ.H.; Erman, F.; Kilic, N.; Aydin, S.; İlhan, N.; İlhan, N.; Ozkan, Y.; Akpolat, N. A comparison of leptin and ghrelin levels in plasma and saliva of young healthy subjects. Peptides 2005, 26, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Ramos, P.S.; Vianna, L.C.; Ricardo, D.R. Heart rate variability across the menstrual cycle in young women taking oral contraceptives. Psychophysiology 2015, 52, 1451–1455. [Google Scholar] [CrossRef]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Non-normal data: Is ANOVA still a Valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [PubMed]
- Berger, B.G.; Motl, R.W. Exercise and mood: A selective review and synthesis of research employing the profile of mood states. J. Appl. Sport Psychol. 2000, 12, 69–92. [Google Scholar] [CrossRef]
- Pretty, J.; Peacock, J.; Hine, R.; Sellens, M.; South, N.; Griffin, M. Green exercise in the UK countryside: Effects on health and psychological well-being, and implications for policy and planning. J. Environ. Plan. Manag. 2007, 50, 211–231. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Bi, S.; Cao, Y.; Zhang, G. Psychological benefits of green exercise in wild or urban greenspaces: A meta-analysis of controlled trials. Urban For. Urban Green. 2022, 68, 127458. [Google Scholar] [CrossRef]
- Rogerson, M.; Brown, D.; Sandercock, G.; Wooller, J.; Barton, J. A comparison of four typical green exercise environments and prediction of psychological health outcomes. Perspect. Public Health 2016, 136, 171–180. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Reimers, A.K.; Knapp, G.; Reimers, C.D. Effects of exercise on the resting heart rate: A systematic review and meta-analysis of interventional studies. J. Clin. Med. 2018, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- Sohn, A.J.; Hasnain, M.; Sinacore, J.M. Impact of exercise (walking) on blood pressure levels in African American adults with newly diagnosed hypertension. Ethn. Dis. 2007, 17, 503–507. [Google Scholar] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Ishii, H.; Furuhashi, S.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) in a mixed forest in Shinano Town, Japan. Scand. J. For. Res. 2008, 23, 278–283. [Google Scholar] [CrossRef]
- Kobayashi, H.; Song, C.; Ikei, H.; Park, B.; Kagawa, T.; Miyazaki, Y. Combined Effect of Walking and Forest Environment on Salivary Cortisol Concentration. Front. Public Health 2019, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Mitsui, M.; Togashi, K.; Matsui, J.; Kato, T.; Uei, D.; Shibayama, A.; Yamato, K.; Okumura, H. Relaxation Effect of a 2-Hour Walk in Kumano-Kodo Forest. J. Neurol. Neurosci. 2017, 8, 174. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D. Salivary Cortisol in Psychobiological Research: An Overview. Neuropsychobiology 1989, 22, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; Longman, D.P. Advancing nature therapy through an evolutionary perspective. School of Sport, Exercise, and Health Science, Loughborough University, Loughborough LE11 3TU, UK. 2023; submitted (manuscript in preparation; to be submitted). [Google Scholar]
- Longman, D.P.; Shaw, C. Human evolutionary ecophysiology: Applying environmental mismatch to investigate human adaptation. School of Sport, Exercise, and Health Science, Loughborough University, Loughborough LE11 3TU, UK. 2023; manuscript in preparation; to be submitted. [Google Scholar]
- Olafsdottir, G.; Cloke, P.; Schulz, A.; Van Dyck, Z.; Eysteinsson, T.; Thorleifsdottir, B.; Vögele, C. Health Benefits of Walking in Nature: A Randomized Controlled Study Under Conditions of Real-Life Stress. Environ. Behav. 2020, 52, 248–274. [Google Scholar] [CrossRef]
- Antonelli, M.; Barbieri, G.; Donelli, D. Effects of forest bathing (shinrin-yoku) on levels of cortisol as a stress biomarker: A systematic review and meta-analysis. Int. J. Biometeorol. 2019, 63, 1117–1134. [Google Scholar] [CrossRef]
- Kalajas-Tilga, H.; Koka, A.; Hein, V.; Tilga, H.; Raudsepp, L. Motivational processes in physical education and objectively measured physical activity among adolescents. J. Sport Health Sci. 2020, 9, 462–471. [Google Scholar] [CrossRef]
- Scott, E.E.; LoTemplio, S.B.; McDonnell, A.S.; McNay, G.D.; Greenberg, K.; McKinney, T.; Uchino, B.N.; Strayer, D.L. The autonomic nervous system in its natural environment: Immersion in nature is associated with changes in heart rate and heart rate variability. Psychophysiology 2021, 58, e13698. [Google Scholar] [CrossRef]
- Schopf, J. The first billion years: When did life emerge? Elements 2006, 2, 229–233. [Google Scholar] [CrossRef]
- Brunet, M.; Guy, F.; Pilbeam, D.; Mackaye, H.T.; Likius, A.; Ahounta, D.; Beauvilain, A.; Blondel, C.; Bocherens, H.; Boisserie, J.-R.; et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature 2002, 418, 145–151. Available online: https://www.nature.com/articles/nature00879 (accessed on 18 February 2023). [CrossRef]
- Hublin, J.J.; Ben-Ncer, A.; Bailey, S.E.; Freidline, S.E.; Neubauer, S.; Skinner, M.M.; Bergmann, I.; Le Cabec, A.; Benazzi, S.; Harvati, K.; et al. New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature 2017, 546, 289–292. [Google Scholar] [CrossRef] [PubMed]
- White, M. The Rise of Cities in the 18th Century; British Library: London, UK, 2009; Available online: https://www.bl.uk/georgian-britain/articles/the-rise-of-cities-in-the-18th-century. (accessed on 18 February 2023).
- Godfrey, R.; Julien, M. Urbanisation and health. Clin. Med. J. R. Coll. Physicians Lond. 2005, 5, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Peen, J.; Schouvers, R.; Beekman, A.; Dekker, J. The current status of urban-rural differences in psychiatric disorders. Acta Psych 2010, 121, 84–93. [Google Scholar] [CrossRef]
- Brod, C. Technostress: The Human Cost of the Computer Revolution; Addison-Wesley: Reading, MA, USA, 1984. [Google Scholar]
- Martins, C.; Kulseng, B.; King, N.A.; Holst, J.J.; Blundell, J.E. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J. Clin. Endocrinol. Metab. 2010, 95, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- McIver, V.J.; Mattin, L.; Evans, G.H.; Yau, A.M.W. The effect of brisk walking in the fasted versus fed state on metabolic responses, gastrointestinal function, and appetite in healthy men. Int. J. Obes. 2019, 43, 1691–1700. [Google Scholar] [CrossRef]
- Jequier, E. Leptin Signaling, Adiposity, and Energy Balance. Ann. N. Y. Acad. Sci. 2002, 6, 379–388. [Google Scholar] [CrossRef] [PubMed]

| Nature | Green Urban | |||
|---|---|---|---|---|
| Baseline | Post | Baseline | Post | |
| TMD * | 91.0 (10.5) | 82.6 (9.0) | 95.4 (9.4) | 89.7 (9.0) |
| Tension | 2.6 (3.4) | 1.2 (1.7) | 2.7 (2.9) | 1.8 (2.3) |
| Anger | 1.0 (1.5) | 0.4 (0.8) | 1.1 (1.5) | 0.5 (1.3) |
| Fatigue | 3.9 (2.5) | 2.1 (2.1) | 4.6 (3.0) | 3.3 (2.9) |
| Depression * | 0.6 (1.1) | 0.2 (0.6) | 1.3 (1.8) | 0.9 (1.8) |
| Confusion * | 2.1 (1.9) | 0.8 (1.2) | 3.0 (2.9) | 1.4 (1.7) |
| Vigour | 5.4 (4.0) | 7.2 (4.3) | 4.5 (3.2) | 5.5 (2.8) |
| ERA * | 13.7 (3.9) | 14.8 (4.2) | 12.9 (3.2) | 12.6 (2.8) † |
| Nature | Green Urban | |||
|---|---|---|---|---|
| Baseline | Post | Baseline | Post | |
| Resting Heart Rate (beats/min) *^ | 63.3 (9.4) | 59.7 (9.3) | 60.6 (11.1) | 57.5 (9.8) |
| Systolic BP (mmHg) * | 108.7 (8.7) | 106.7 (8.6) | 109.7 (8.9) | 107.1 (9.3) |
| Diastolic BP (mmHg) * | 65.4 (5.4) | 64.6 (5.1) | 66.2 (7.5) | 65.0 (7.0) |
| Cortisol (nmol mL−1) * | 14.0 (7.4) | 7.5 (5.4) | 16.1 (10.4) | 6.3 (3.2) |
| Nature | Green Urban | ||
|---|---|---|---|
| Post | Post | Post-Nature vs. Post-Green Urban t/z | |
| Total energy intake (kJ) | 3138 (923) | 3206 (1037) | −0.54 a |
| Savoury (kJ) | 2401 (893) | 2446 (915) | −0.29 a |
| Sweet (kJ) | 740 (337) | 760 (405) | −0.78 a |
| Sweet:savoury ratio | 0.4 (0.6) | 0.4 (0.3) | −0.11 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, Y.; Wellings, I.; Thompson, H.; Barutcu, A.; James, L.; Bishop, N.; O’Donnell, E.; Shaw, C.; Longman, D.P. Additional Health Benefits Observed following a Nature Walk Compared to a Green Urban Walk in Healthy Females. Urban Sci. 2023, 7, 85. https://doi.org/10.3390/urbansci7030085
Todorova Y, Wellings I, Thompson H, Barutcu A, James L, Bishop N, O’Donnell E, Shaw C, Longman DP. Additional Health Benefits Observed following a Nature Walk Compared to a Green Urban Walk in Healthy Females. Urban Science. 2023; 7(3):85. https://doi.org/10.3390/urbansci7030085
Chicago/Turabian StyleTodorova, Yvanna, Izzy Wellings, Holly Thompson, Asya Barutcu, Lewis James, Nicolette Bishop, Emma O’Donnell, Colin Shaw, and Daniel P. Longman. 2023. "Additional Health Benefits Observed following a Nature Walk Compared to a Green Urban Walk in Healthy Females" Urban Science 7, no. 3: 85. https://doi.org/10.3390/urbansci7030085
APA StyleTodorova, Y., Wellings, I., Thompson, H., Barutcu, A., James, L., Bishop, N., O’Donnell, E., Shaw, C., & Longman, D. P. (2023). Additional Health Benefits Observed following a Nature Walk Compared to a Green Urban Walk in Healthy Females. Urban Science, 7(3), 85. https://doi.org/10.3390/urbansci7030085







