Abstract
Diverse larval habitats significantly influence female mosquito oviposition. Utilizing traps that simulate these habitats is helpful in the study of the bioecology and characteristics of pathogen-transmitting species during oviposition. This study evaluated the feasibility of different traps in natural environments by comparing sampling methods and detecting the oviposition of epidemiologically important mosquitoes, with emphasis on Haemagogus species, in a fragment of the Atlantic Forest in Silva Jardim, Rio de Janeiro State, Brazil. Monthly collections were conducted from March 2021 to October 2023 using four types of traps: plastic containers, tires, bamboo, and sapucaia. Immatures were collected from these traps using a pipette, placed in plastic bags, and transported to the laboratory. Tire was the most efficient trap, showing the highest mosquito abundance (n = 1239) and number of species (S = 11). Conversely, the plastic container trap exhibited the lowest diversity (H = 0.43), with only two species and a low mosquito abundance (n = 26). The bamboo trap captured six species and recorded the second-highest diversity index (H = 1.04), while the sapucaia trap captured five species and had the third-highest diversity index (H = 0.91). Of the total immatures collected, 1817 reached adulthood, comprising 13 species, two of which are vectors of the sylvatic yellow fever virus: Haemagogus leucocelaenus and Haemagogus janthinomys. In conclusion, detecting key vectors of the sylvatic yellow fever virus in Brazil highlights the need for ongoing entomological and epidemiological surveillance in the study area and its vicinity. These efforts are crucial for monitoring vector presence and activity, identifying potential transmission hotspots, and devising effective control and prevention strategies.
1. Introduction
Culicidae, insects widely distributed in urban and forest environments, depend on water collections to ensure the successful development of their immature stages (egg, larva, and pupa). These aquatic environments serve as habitats for the immature forms, which, in addition to providing water for oviposition and the continuation of immature phases, are also influenced by chemical and physical factors [1].
Immature habitats are categorized as either natural or artificial. Natural habitats typically include ponds, wetlands, tree hollows, bromeliads, and bamboo, whereas artificial habitats comprise containers that accumulate water, such as dams, water tanks, tires, and cans [2]. Female mosquitoes are selective about their breeding sites. Specifically, those of the genus Haemagogus prefer temporary sites in forest environments, often colonizing tree and bamboo hollows, with records also in bamboo and bromeliad internodes [3,4,5].
Using traps for oviposition by female mosquitoes facilitates data collection on species richness, dominance, abundance, and diversity. These traps also serve as tools for taxonomic studies critical for epidemiological surveillance in regions affected by arboviruses, such as the sylvatic yellow fever virus (YFV). Among the species identified as transmitters of YFV, Haemagogus janthinomys Dyar, 1921 is recognized as the primary vector in the Americas, while Haemagogus leucocelaenus Dyar and Shannon, 1924 was implicated in the recent YFV outbreak from 2016 to 2018 in Rio de Janeiro State, Brazil [6]. During epidemic periods, these species often have high infection rates in tree-canopy samples [7].
The present study aimed to evaluate the feasibility of various types of traps introduced into the natural environment, compare their efficiency, and detect the oviposition of mosquito species of epidemiological importance, with a focus on Haemagogus species in a segment of the Atlantic Forest located in the municipality of Silva Jardim, Rio de Janeiro State.
2. Material and Methods
2.1. Ethics Statement
The permanent license for the collection, capture, and transport of zoological material was granted by the Chico Mendes Institute for Biodiversity Conservation (ICMBio) and the Biodiversity Authorization and Information System (SISBIO) under license number 44333-1, Rio de Janeiro, Brazil. All team members were properly vaccinated against yellow fever.
2.2. Study Area
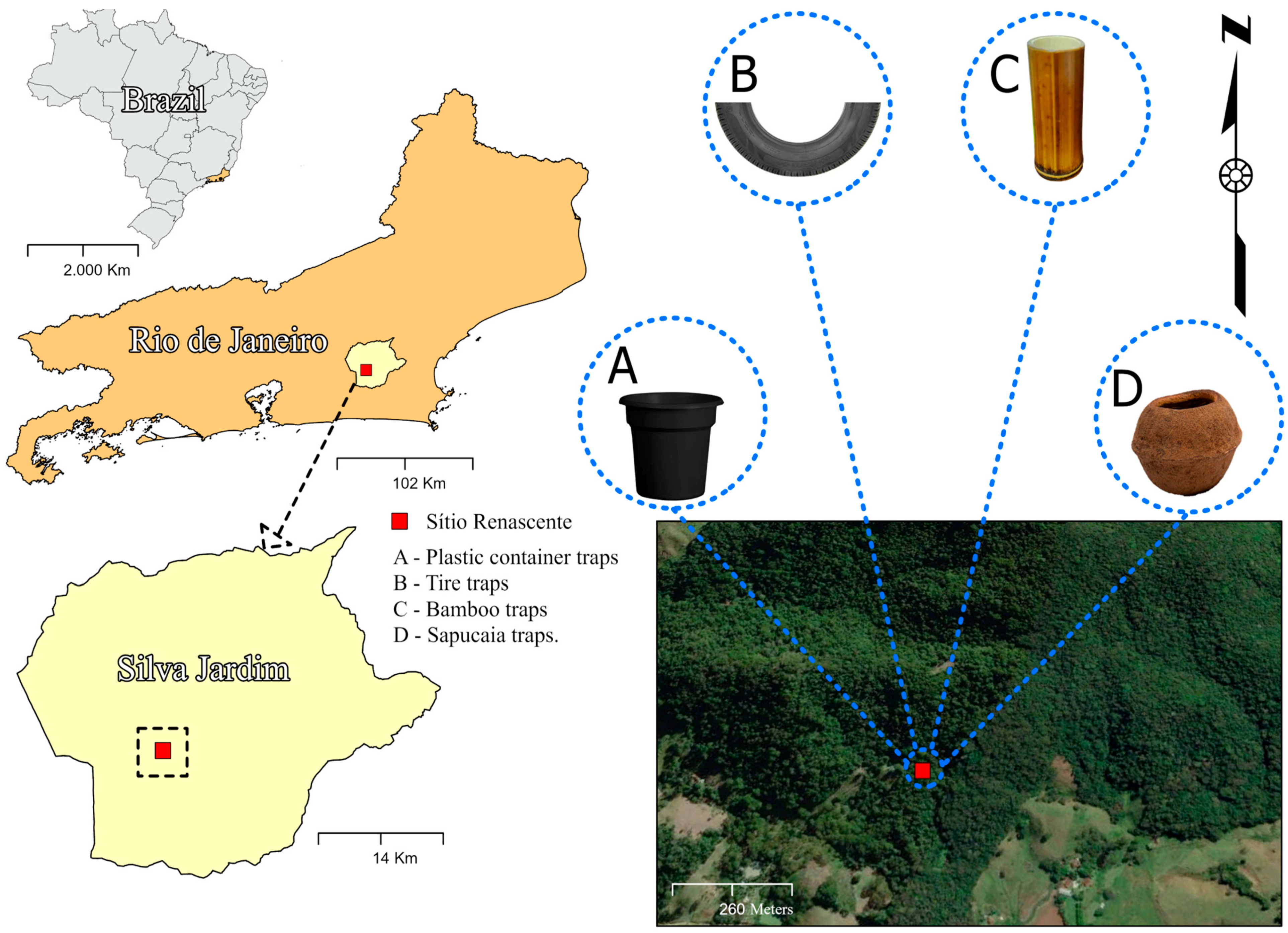
The sampling area is situated within a remnant of the Atlantic Forest, located on the boundaries of the Renascente site in the central–north region of Rio de Janeiro, specifically in Silva Jardim. This region still preserves more than 18.70% of the original Atlantic Forest coverage (Fundação SOS Mata Atlântica, 2019). Sub-mountain dense ombrophilous forest is the predominant vegetation [8]. The regional climate is categorized as a rainy tropical climate with a dry season in winter, according to the Köppen classification. Annual rainfall ranges from 1500 to 2000 mm, with the period from November to March experiencing the highest rainfall and temperatures, and varies annually between 32 and 24 °C [9]. The collection site was located at the geographical coordinates 22°36′49.1″ S 42°27′34.1″ W (Figure 1).
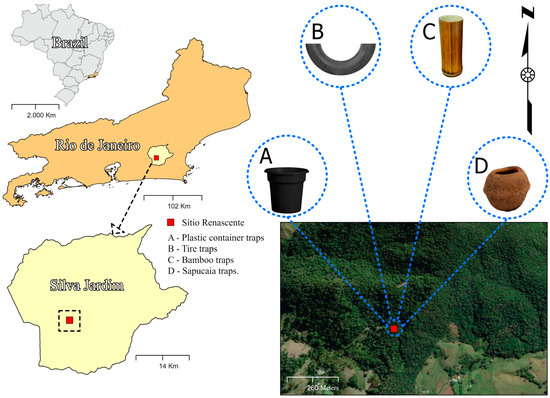
Figure 1.
Map of the study site in the municipality of Silva Jardim, Rio de Janeiro State, Brazil.
2.3. Experimental Design
Collections were conducted monthly from March 2021 to October 2023. The traps for collecting mosquito immatures were characterized as follows. (a) The plastic container traps consisted of a matte black container with a 500 mL water capacity. The container had no lid and was filled with natural water and forest litter (leaves, branches, and other organic materials) to mimic a natural ecosystem. (b) The tire traps were made from a third of a motorcycle tire; these traps served as containers with 1000 mL of water capacity. (c) The bamboo traps were constructed from a bamboo internode; these traps were separated to create a container about 30 cm deep, with an opening approximately 25 cm in diameter and a 1000 mL water capacity. (d) The sapucaia traps were made from the woody fruit of the Atlantic Forest chestnut tree (“monkey-cumbuca”); these traps were 12 to 20 cm in diameter with an operculum or dehiscent lid. For the experiment, a fruit capable of holding 500 mL of water was selected, occupying two-thirds of the fruit’s volume and matching the water volume in the other traps. To collect immature mosquitoes, 500 mL of water were added to each trap. The traps were installed upright and tied to the trunks of four trees at the same location and positioned 2 m from the ground level.
The larvae and pupae found in the traps were collected using a plastic pipette, transferred to 250 mL plastic bags (Whirl-Pak® bags, BioQuip®, Seattle, WA, USA), and transported to the laboratory. In the laboratory, they were maintained alive in pots containing water from the reservoir in which they were collected, supplemented with dechlorinated water as needed due to evaporation until they reached the adult phase. They were kept in an air-conditioned B.O.D. chamber (ELETROlab, EL131/3, capacity: 354 L, São Paulo, Brazil) in controlled conditions, with a temperature of 28 ± 1 °C, relative humidity from 75% to 90%, and a photoperiod of 12 h. The larvae were fed twice weekly with fish food (TetraMin®, Melle, Germany), which was ground and diluted in 10 mL of water.
In the laboratory, the tire, bamboo, plastic container, and sapucaia traps were immersed in plastic basins filled with distilled water to facilitate any potential hatching of the eggs deposited on their walls. Immatures reaching the adult stage were euthanized by intoxication using a solution of ethyl ether or chloroform.
Culicids were identified by direct observation of the morphological characters evident under a stereoscopic microscope (Leica DMD108®, Wetzlar, Germany), based on the dichotomous keys by Lane (1953) [10,11], Faran and Linthicum (1981) [12], Consoli and Lourenço-de-Oliveira (1994) [13], and Forattini (2002) [3]. Following identification, they were deposited in the Entomological Collection of the Oswaldo Cruz Institute, FIOCRUZ, under the title “Mata Atlântica/RJ”.
2.4. Statistical Analyses
Several statistical analyses were conducted to assess the structure of the mosquito community. These included the Shannon diversity index (H), which serves as an indicator of both species richness and evenness; Pielou’s equitability (J), which assesses the equitable distribution of species abundances; species richness (S), to represent the total number of distinct species; absolute abundance of individuals, which indicates the overall count of the mosquitoes; and the dominance index (D), which reveals prevalent patterns within the community. Additionally, the Morisita similarity index was used to quantify the level of resemblance between different mosquito breeding sites. This index aids in elucidating the patterns of species distribution and composition across diverse habitats. All statistical analyses were performed using the software PAST version 4.05, a robust analytical platform for exploring ecological data [14].
3. Results
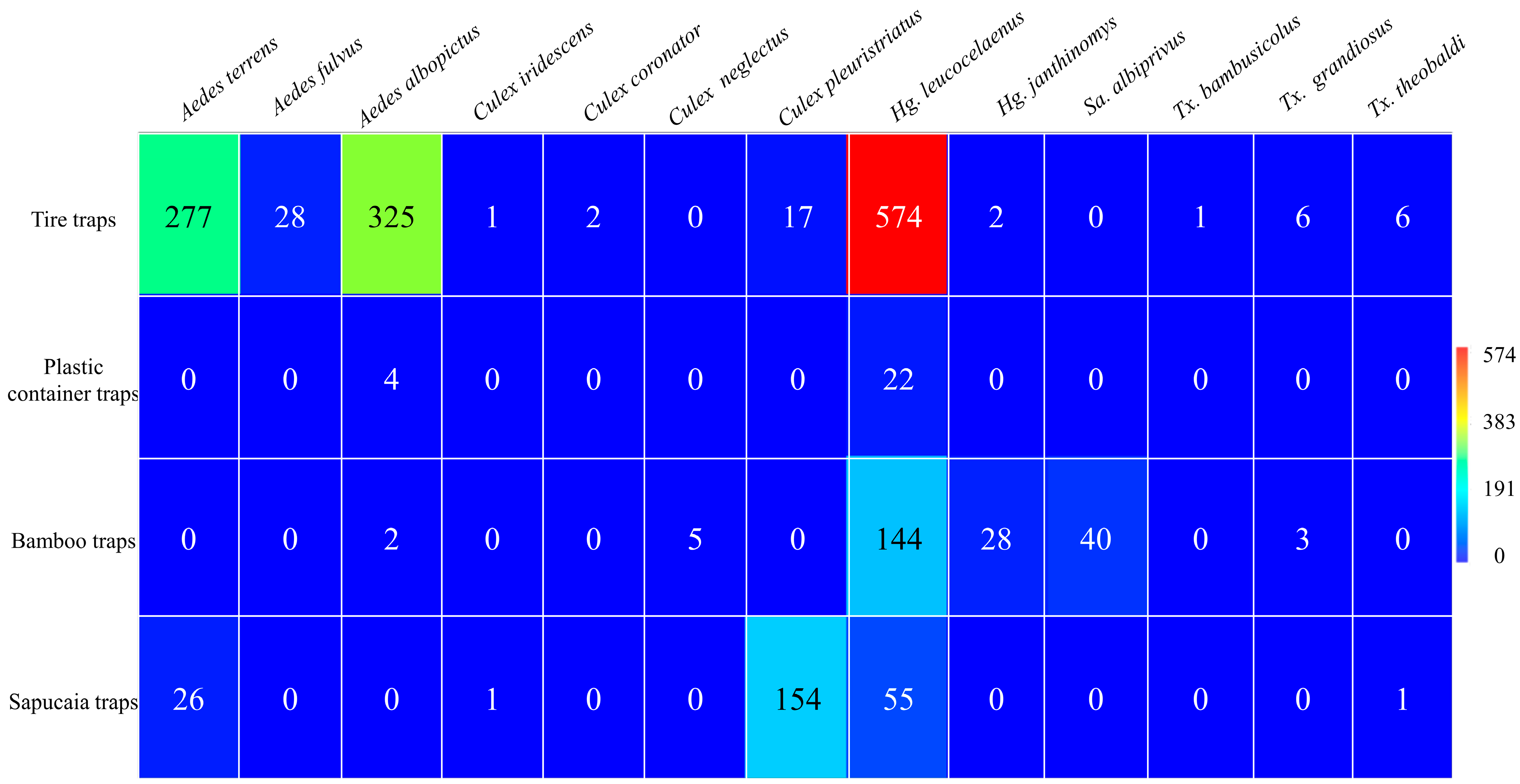
During the sampling period, 2016 immatures were collected, of which 1723 reached adulthood in the types of traps used. The greatest abundance was found in tire traps (n = 1239; 72%), followed by sapucaia traps (n = 237; 14%), bamboo traps (n = 222; 13%), and plastic container traps (n = 26; 2%). The mosquito species recorded and their respective abundances included Aedes terrens Walker, 1856 (n = 303; 17.6%); Aedes fulvus Wiedemann, 1828 (n = 28; 2%); Aedes albopictus Skuse, 1894 (n = 331; 19.2%); Culex iridescens Lutz, 1905 (n = 2; 0.1%); Culex coronator Dyar and Knab, 1906 (n = 2; 0.1%); Culex neglectus Lutz, 1904 (n = 5; 0.3%); Culex pleuristriatus Theobald, 1903 (n = 171; 9.9%); Haemagogus leucocelaenus (n = 795; 46.1%); Haemagogus janthinomys (n = 30; 1.7%); Sabethes albiprivus Theobald, 1903 (n = 40; 2.3%); Toxorhynchites bambusicolus Lutz and Neiva 1913 (n = 1; 0.1%); Toxorhynchites cf. grandiosus Williston 1900 (n = 9; 0.5%); and Toxorhynchites cf. theobaldi Dyar and Knab, 1906 (n = 70; 4%) (Figure 2).
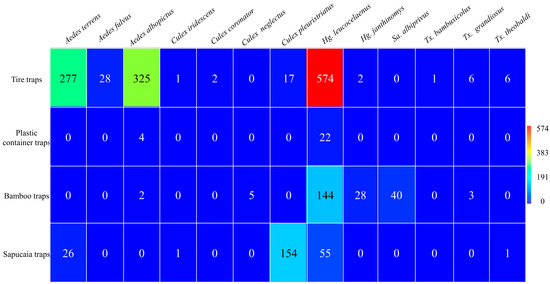
Figure 2.
Abundance of mosquitoes collected from different traps at Sítio Renascente, Silva Jardim, Rio de Janeiro State, Brazil, from March 2021 to October 2023.
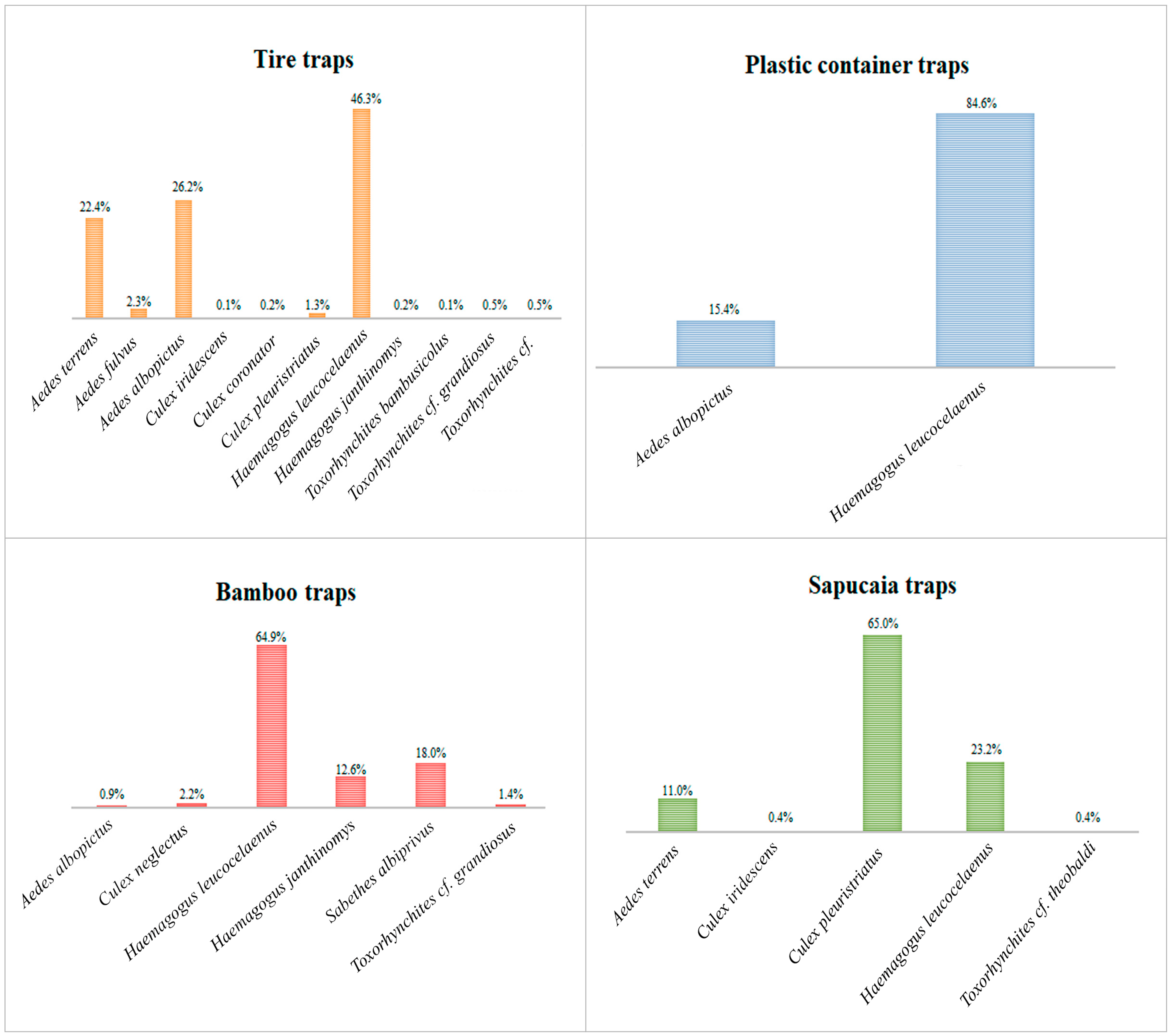
Analyzing the species separately by trap, we observed that Hg. leucocelaenus exhibited greater abundance in all traps, except the sapucaia traps, where it was the second most abundant species. Haemagogus leucocelaenus accounted for 85% of the total adult specimens in the plastic container traps, 65% in the bamboo traps, 46% in the tire traps, and 23% in the sapucaia traps. Meanwhile, Hg. janthinomys was present in the bamboo and tire traps, representing 13% and 0.2% of their respective populations (Figure 3).
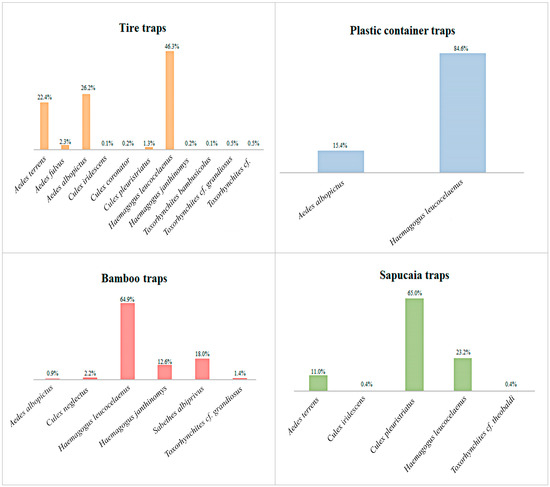
Figure 3.
Percentage of the abundance of each species per trap, considering all Culicidae species collected at Sítio Renascente, Silva Jardim, Rio de Janeiro State, Brazil, from March 2021 to October 2023.
Tires stood out as the most prolific traps, exhibiting the highest abundance of mosquitoes and species richness (S = 11). Additionally, they presented a high diversity index (H = 1.27), indicating a wide variety of species present. However, their equitability was relatively low (J = 0.53), suggesting an unequal distribution among species. In contrast, the plastic container traps had the lowest diversity (H = 0.43), registering only two species and a low mosquito abundance (26 individuals). These traps showed a considerable dominance index (D = 0.73), with most specimens belonging to Hg. leucocelaenus (85%). Six species were reported in the bamboo traps, presenting the second-highest diversity index (H = 1.04). Finally, five species were recorded in the sapucaia traps, which exhibited the third-highest diversity index (H = 0.91) (Table 1, Supplementary File S1).

Table 1.
Ecological indices of the different traps installed at Sítio Renascente, Silva Jardim, Rio de Janeiro State, Brazil, from March 2021 to October 2023.
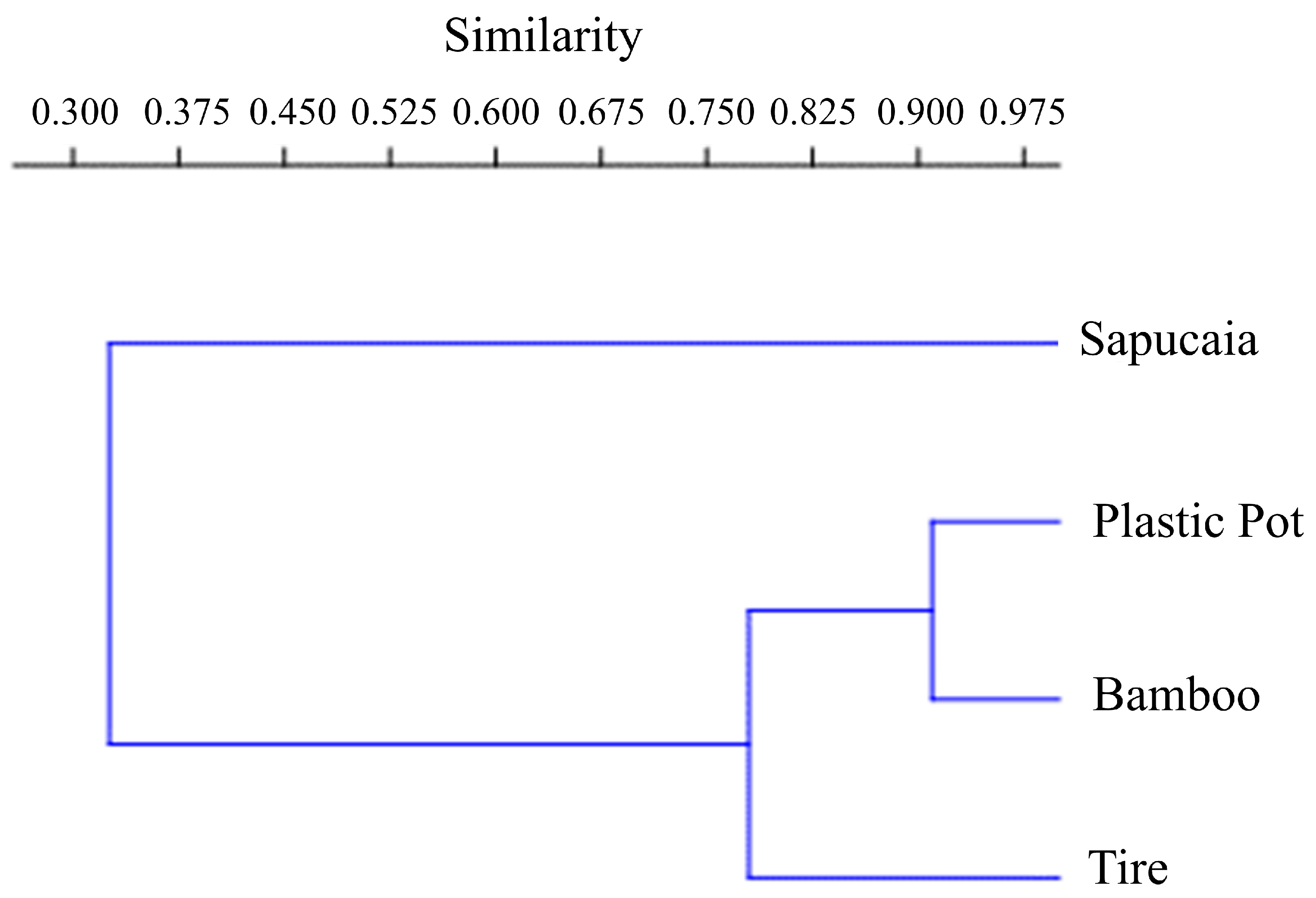
According to the Morisita similarity index, the sapucaia traps were the most divergent, differing significantly from the others. These recorded a higher number of Cx. pleuristriatus individuals. The most similar traps were the plastic container and the bamboo traps, both featuring similar equitability indices (bamboo traps, J = 0.5786; plastic container traps J = 0.6194) (Figure 4). Both types of traps predominantly captured Hg. leucocelaenus, accounting for 85% of the Culicidae in the plastic container traps and 65% in the bamboo traps.
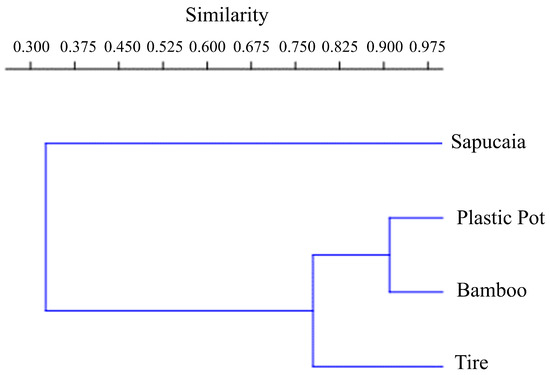
Figure 4.
Morisita index cladogram of similarity between mosquito immature traps at Sítio Renascente, Silva Jardim, Rio de Janeiro State, Brazil, from March 2021 to October 2023.
4. Discussion
Culicidae immatures inhabit a variety of water-collecting environments, featuring diverse substrates with varying degrees of human influence. This plasticity highlights the importance of conducting studies aimed at evaluating, characterizing, and defining different ecological variables across various traps. Several species exhibit significant ecological flexibility, enabling them to thrive in varied environments that support their progression from immature to adult stages [3].
Maia et al. (2020) [15] employed three types of traps akin to those in this study: tire, bamboo, and plastic container traps. The tire traps captured the largest number of individuals, which is in line with our observations. However, there was a variance in the most abundant species; their study reported significantly lower proportions of Hg. leucocelaenus (5.90% of total individuals at collection point 1 and 1.30% at collection point 2), compared to our findings, which indicated a much higher prevalence, nearly 47%.
We found that tires were the most effective traps, capturing the highest abundance of immature Culicidae and the largest number of Hg. leucocelaenus. In Brazil, similar studies comparing natural and artificial traps in the rural areas of northern Paraná also reported the highest mosquito abundance in tire traps [16,17]. Aedes albopictus was the second most abundant species in the tire traps, a similar finding to that of Albuquerque et al. (2000) [18], who noted a high abundance of Ae. albopictus in tires in a remnant of the Atlantic Forest in the urban area of Recife, Pernambuco. In our study, the species found with the highest abundance in the tire traps were Hg. leucocelaenus (46%), Ae. albopictus (26%), and Ae. terrens (22%). These results contrast with those from Nova Iguaçu, Rio de Janeiro, where the most abundant species in the tire traps were Limatus durhamii Theobald, 1901 (57.8%) and Limatus pseudomethisticus Bonne-Wepster and Bonner, 1920 (25.7%), and in Londrina, Paraná, where the predominant species in the tire traps were Culex eduardoi Casal and García, 1968, Culex laticlasper Galindo and Blanton, 1954, Culex bigoti Bellardi, 1862, and Culex quinquefasciatus Say, 1823 [15,16].
In the present study, the bamboo traps exhibited the second-highest index of Culicidae diversity. These results are in line with those from a study conducted on live bamboo internodes in Nagasaki, Japan, where the most dominant arthropod groups were immature stages of Diptera from the families Ceratopogonidae and Culicidae [19]. The prevalence of immature Culicidae in bamboo underscores the role of plants as habitats for various mosquito species, including those of public health relevance. Our study revealed a significant abundance of Hg. leucocelaenus (65%) in bamboo traps, underscoring its relevance as a medically important species and a YFV vector [20,21].
The high diversity observed in bamboo traps is also supported by Bastos et al. (2021) [22]. They conducted a study on the composition of Culicidae that breed in internodes of Bambusa sp., finding a richness of 17 species in a remnant of Atlantic Forest in Rio de Janeiro State. In our study, six species were recorded in this trap type, the most abundant being Hg. leucocelaenus (65%), Sa. albiprivus (18%), and Hg. janthinomys (13%). These genera are of extreme medical significance, as they are the principal sylvatic vectors of YFV in the Americas [4,7,23].
We observed that the plastic container and bamboo traps showed high similarity, as evidenced by the Morisita index. This similarity was further supported by comparable equitability indices, indicating an equitable distribution of the species present. Additionally, both traps exhibited a high abundance of Hg. leucocelaenus and Ae. albopictus, suggesting that they may serve as favorable breeding sites for these pathogen-transmitting vectors. These traps provide similar and favorable environmental conditions for Culicidae proliferation. They feature a cylindrical shape, which offers good water storage capacity, potentially reducing evaporation. Moreover, both can accumulate organic debris, such as leaves and plant remains, serving as a food source for immature forms.
The presence of other traps installed at the sampling point may have influenced oviposition, as these species already recognize them as potentially advantageous larval breeding habitats. The two species found most abundantly in these traps (plastic container and bamboo traps) display strong adaptive capacity, enabling them to colonize traps in both forest environments and those modified by human activity [4].
Marques and Forattini (2008) [24] noted that the abundance of species reflects the control one species exerts over others to become dominant. The dominance of Hg. leucocelaenus and Ae. albopictus, found in nearly all traps, is likely due to their biological, ecological, and behavioral similarities. Both species are sylvatic, laying their eggs on moist substrates near the water surface; they are typically found in tropical and subtropical forest environments, with Ae. albopictus also prevalent in rural areas [3,25,26,27].
Understanding the diversity of insects in the Atlantic Forest is crucial for assessing changes in population activity patterns. Our results suggest that sampling methods for collecting immature mosquitoes are significant indicators in this regard. Tire traps showed the highest abundance and richness of Culicidae, underscoring their importance in projects monitoring biological vectors relevant to public health. The increase in production and improper disposal of materials such as tires significantly contributes to the creation of mosquito breeding sites. Aedes aegypti Linnaeus, 1762, a vector for diseases like dengue, Zika, and chikungunya, notably thrives in these environments. Tires are particularly effective breeding sites for mosquitoes due to their water retention capacity and protection for larval development. Moreover, they can act as passive carriers of mosquito eggs and larvae, potentially being moved by rainwater or human actions, thus spreading mosquitoes to new areas and contributing to disease transmission [28]. Consequently, tire traps for collecting immature mosquitoes are valuable tools in fauna surveys, functioning as artificial phytotelmata [29].
5. Conclusions
The observed oviposition behavior of mosquito species of the genus Haemagogus, specifically Hg. leucocelaenus and Hg. Janthinomys, demonstrates their capacity to lay eggs in artificial containers. This behavior increases the risk of contact between humans and vectors at the study site. Consequently, using various traps for sampling is an effective approach for surveying Culicidae fauna, enabling studies focused on the surveillance and monitoring of medically important vectors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed9060125/s1. Supplementary File S1: Diversity and abundance of species mosquitoes collected from different traps at Sítio Renascente, Silva Jardim, Rio de Janeiro State, Brazil, from March 2021 to October 2023.
Author Contributions
Conceptualization: R.D., A.L.C.-d.-l.-F. and J.A. Formal analysis: S.O.F.S. Methodology: R.D., C.F.d.M., H.R.G.-S. and J.A. Supervision: J.A. Writing—original draft: R.D., C.F.d.M., S.O.F.S., H.R.G.-S., A.L.C.-d.-l.-F. and J.A. Writing—review and editing: R.D., C.F.d.M., S.O.F.S., A.L.C.-d.-l.-F. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of CNPq, FAPERJ, and CAPES, as well as the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant number: 303286/2021-0, J.A.) and the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/200.956/2002/2022, J.A.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Hecio Gomes and Tatiana Gomes for work support at the Renascente site in the central–north region of Rio de Janeiro, municipality of Silva Jardim, Rio de Janeiro State, Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bentley, M.D.; Day, J.F. Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 1989, 34, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.G. Os mosquitos (Diptera, Culicidae) e a sua importância médica em Portugal—Desafios para o Século XXI. Acta Médica Port. 2011, 24, 961–974. [Google Scholar] [CrossRef]
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia, Epidemiologia; Edusp—Editora da Universidade de São Paulo: São Paulo, Brasil, 2002. [Google Scholar]
- Marcondes, C.; Alencar, J. Revisão de mosquitos Haemagogus Williston (Diptera: Culicidae) do Brasil. Rev. Biomédico 2010, 21, 221–238. [Google Scholar]
- Leite, M.P.C.; Dias, R.; Leite, P.J.; Silva, S.O.F.; Gil-Santana, H.R.; Barbosa, R.P.; de Mello, C.F.; Alencar, J. Bamboo (Poales, Poaceae): An Important Maintainer of Immature Mosquitoes (Diptera: Culicidae) in a Biodiversity Hotspot in the City of Rio de Janeiro, Brazil. Life 2024, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, F.V.S.; de Andreazzi, C.S.; Neves, M.S.A.S.; Meneguete, P.S.; Ribeiro, M.S.; Dias, C.M.G.; de Albuquerque Motta, M.; Barcellos, C.; Romão, A.R.; Magalhães, M.d.A.F.M.; et al. Ecological and environmental factors affecting transmission of sylvatic yellow fever in the 2017–2019 outbreak in the Atlantic Forest, Brazil. Parasites Vectors 2022, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.C. Febre amarela. Rev. Soc. Bras. Med. Trop. 2003, 36, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Veloso, H.P.; Rangel-Filho, A.L.R.; Lima, J.C.A. Classificação da Vegetação Brasileira, Adaptada a um Sistema Universal; IBGE: Rio de Janeiro, Brazil, 1991; p. 124.
- Primo, P.B.; Volker, C.M. Bacias Hidrográficas dos Rios São João e das Ostras: Águas, Terras e Conservação Ambiental; Consórcio Intermunicipal Lagos São João: Rio de Janeiro, Brazil, 2003; p. 19. [Google Scholar]
- Lane, J. Neotropical Culicidae; Universidade de São Paulo: São Paulo, Brasil, 1953; Volume 1. [Google Scholar]
- Lane, J. Neotropical Culicidae; Universidade de São Paulo: São Paulo, Brasil, 1953; Volume 2. [Google Scholar]
- Faran, M.E.; Linthicum, K.J. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq. Syst. 1981, 13, 1–81. [Google Scholar]
- Consoli, R.A.G.B.; Lourenço-De-Oliveira, R. Principais Mosquitos de Importância Sanitária do Brasil; Editora FIOCRUZ: Rio de Janeiro, Brasil, 1994. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological statistics software package for education and data analysis past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Aguiar, M.D.; Bastos, A.Q.; Leite, P.J.; Gil-Santana, H.R.; Santos, S.J.; Alencar, J. Comparative Analysis Between Sampling Methods for Immature Mosquitoes in an Atlantic Forest Fragment in Brazil. J. Am. Mosq. Control Assoc. 2020, 4, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J. Mosquito (Diptera: Culicidae) ecology of natural and artificial rural breeding places in northern Parana, Brazil. V. Larvae captured in artificial reservoirs installed in ciliary. Rev. Saude Publica 1997, 31, 370–377. [Google Scholar] [CrossRef]
- Zequi, A.C.; Lopes, J.; Medri, I.M. Imaturos de Culicidae (Diptera) encontrados em recipientes instalados em mata residual no município de Londrina, Paraná, Brasil. Rev. Bras. Zool. 2005, 22, 656–661. [Google Scholar] [CrossRef]
- Albuquerque, C.M.; De Melo-Santos, M.A.V.; Bezerra, M.A.S.; Barbosa, R.M.; Silva, D.F.; Silva, E. Primeiro registro de Aedes albopictus em área da Mata Atlântica, Recife, PE, Brasil. Rev. Saude Publica 2000, 34, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Motoyoshi, M.; Hiroshi, S. The Biotic Community in the Water-Filled Internode of Bamboos in Nagasaki, Japan, with Special Reference to Mosquito Ecology. Jpn. J. Ecol. 1978, 33, 271–279. [Google Scholar] [CrossRef]
- Aragão, N.C.; Müller, G.A.; Balbino, V.Q.; Costa Junior, C.R.L.; Figueirêdo Júnior, C.S.; Alencar, J.; Marcondes, C.B. A list of mosquito species of the Brazilian State of Pernambuco, including the first report of Haemagogus janthinomys (Diptera: Culicidae), yellow fever vector and 14 other species (Diptera: Culicidae). Rev. Soc. Bras. Med. Trop. 2010, 43, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.D.; Miranda, R.M.; Bonelly, I.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.D.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.Q.; Leite, P.J.; Mello, C.F.; Maia, D.A.; Machado, S.L.; Gil-Santana, H.R.; Silva, S.O.F.; dos Santos-Mallet, J.R.; Alencar, J. Bionomy of Mosquitoes in Bamboo Internodes in an Atlantic Forest Remnant of the State of Rio De Janeiro, Brazil. J. Am. Mosq. Control Assoc. 2021, 37, 208–215. [Google Scholar] [CrossRef]
- Goenaga, S.; Fabbri, C.; Dueñas, J.C.R.; Gardenal, C.N.; Rossi, G.C.; Calderon, G.; Morales, M.A.; Garcia, J.B.; Enria, D.A.; Levis, S. Isolation of yellow fever virus from mosquitoes in Misiones Province, Argentina. Vector-Borne Zoonotic Dis. 2012, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.R.A.M.; Forattini, O.P. Culicídeos em bromélias: Diversidade de fauna segundo influência antrópica, litoral de São Paulo. Rev. Saúde Pública 2008, 42, 979–985. [Google Scholar] [CrossRef]
- Correa, F.F.; Gleiser, R.M.; Leite, P.J.; Fagundes, E.; Gil-Santana, H.R.; Mello, C.F.; Gredilha, R.; Alencar, J. Mosquito communities in Nova Iguaçu Natural Park, Rio de Janeiro, Brazil. J. Am. Mosq. Control Assoc. 2014, 30, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.O.F.; Mello, C.F.; Figueiró, R.; Maia, D.A.; Alencar, J. Distribution of the Mosquito Communities (Diptera: Culicidae) in Oviposition Traps Introduced into the Atlantic Forest in the State of Rio de Janeiro, Brazil. Vector-Borne Zoonotic Dis. 2018, 18, 214–221. [Google Scholar] [CrossRef]
- Müller, G.A.; de Mello, C.F.; Bueno, A.S.; de Alcantara Azevedo, W.T.; Alencar, J. Little noticed, but very important: The role of breeding sites formed by bamboos in maintaining the diversity of mosquitoes (Diptera: Culicidae) in the Atlantic Forest biome. PLoS ONE 2022, 17, e0273774. [Google Scholar] [CrossRef] [PubMed]
- Tauil, P. Aspectos críticos do controle do dengue no Brasil. 62. Cad. Saúde Pública 2002, 18, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Tátila-Ferreira, A.; Maia, D.D.A.; De Abreu, F.V.S.; Rodrigues, W.C.; Alencar, J. Oviposition behavior of Haemagogus leucocelaenus (Diptera: Culicidae), a vector of wild yellow fever in Brazil. Rev. Inst. Med. Trop. 2017, 59, e60. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).