Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance
Abstract
:1. Introduction
2. Dietary Sources of Butyrate
2.1. Milk
2.2. Dietary Fiber
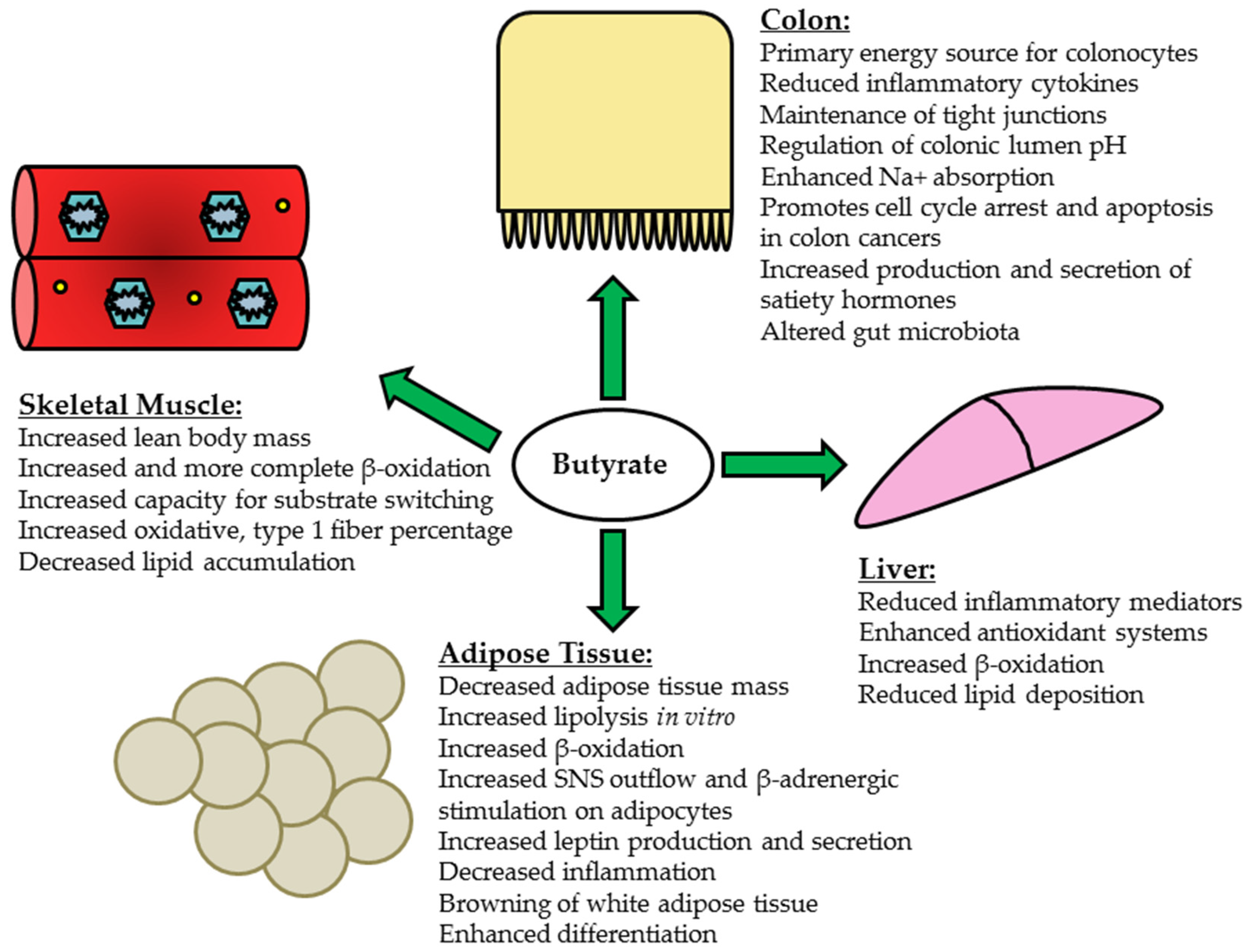
3. Butyrate in the Gastrointestinal Tract
3.1. Effects of Microbiota on Short Chain Fatty Acid Synthesis in the Gut
3.2. Mechanisms of Butyrate Uptake and Action in the Gut
3.3. Chemopreventive Effects of Butyrate
3.4. Anti-Inflammatory Effects of Butyrate
4. Butyrate, Obesity and T2D
4.1. Butyrate and Obesity
4.2. Butyrate and Insulin Resistance
5. Butyrate and Peripheral Tissues
5.1. Liver
5.2. Skeletal Muscle
5.3. Adipose Tissue
6. Butyrate and Clinical Studies
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keenan, M.J.; Zhou, J.; Hegsted, M.; Pelkman, C.; Durham, H.A.; Coulon, D.B.; Martin, R.J. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv. Nutr. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Heyer, C.M.; Messner, S.; Rings, A.; Louis, S.; Bischoff, S.C.; Mosenthin, R. Impact of a High-Fat or High-Fiber Diet on Intestinal Microbiota and Metabolic Markers in a Pig Model. Nutrients 2016, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Agro, N.C.; Eliasson, A.M.; Mialki, K.L.; Olivera, J.D.; Rusch, C.T.; Young, C.N. Health Benefits of Fiber Fermentation. J. Am. Coll. Nutr. 2017, 36, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.J.; Jonkers, D.M. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Henagan, T.M.; Stefanska, B.; Fang, Z.; Navard, A.M.; Ye, J.; Lenard, N.R.; Devarshi, P.P. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br. J. Pharmacol. 2015, 172, 2782–2798. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485s–2493s. [Google Scholar] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, S.N.; Kala, R.; Tollefsbol, T.O. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp. Cell Res. 2014, 324, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J.; et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring) 2006, 14, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; Shen, L.; Lissy, E.; McCutcheon, K.; Keenan, M.J. Failure to ferment dietary resistant starch in specific mouse models of obesity results in no body fat loss. J. Agric. Food Chem. 2009, 57, 8844–8851. [Google Scholar] [CrossRef] [PubMed]
- Vidrine, K.; Ye, J.; Martin, R.J.; McCutcheon, K.L.; Raggio, A.M.; Pelkman, C.; Durham, H.A.; Zhou, J.; Senevirathne, R.N.; Williams, C.; et al. Resistant starch from high amylose maize (HAM-RS2) and dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity (Silver Spring) 2014, 22, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-coupled Receptors GPR 41 and GPR 43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Su, H.; Zhou, Z.; Yao, W. Identification of the Porcine G Protein-Coupled Receptor 41 and 43 Genes and Their Expression Pattern in Different Tissues and Development Stages. PLoS ONE 2014, 9, e97342. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Yano, N.; Wang, H.; Zhao, Y.T.; Dubielecka, P.M.; Zhuang, S.; Chin, Y.E.; Qin, G.; Zhao, T.C. Sodium Butyrate Protects-Against High Fat Diet-Induced Cardiac Dysfunction and Metabolic Disorders in Type II Diabetic Mice. J. Cell. Biochem. 2017, 8, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Sleeth, M.L.; Thompson, E.L.; Ford, H.E.; Zac-Varghese, S.E. Frost G Free fatty acid receptor 2 and nutrient sensing: A proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr. Res. Rev. 2010, 23, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food. Sci. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Parodi, P.W. Fatty acid composition of australian butter and milk fats. Aust. J. Dairy Technol. 1970, 25, 200–205. [Google Scholar]
- Marshall, M.O.; Knudsen, J. The biosynthesis of short-chain triacylglycerols by microsomal fractions from lactating-cow mammary gland. Biochem. Soc. Trans. 1977, 5, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Kuksis, A.; Marai, L.; Myher, J.J. Triglyceride structure of milk fats. J. Am. Oil Chem. Soc. 1973, 50, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Karupaiah, T.; Sundram, K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: A review of their nutritional implications. Nutr. Metab. 2007, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W.; Clapperton, J.L. Structures of the triglycerides of cows’ milk, fortified milks (including infant formulae), and human milk. J. Soc. Dairy Technol. 1982, 35, 22–24. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhong, X.; He, J.; Zhang, L.; Bai, K.; Xu, W.; Wang, T.; Huang, X. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin. Nutr. 2016, 35, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Savary, G.; Jaguelin-Peyrault, Y.; Rome, V.; Le Normand, L.; Zabielski, R. Dietary sodium butyrate supplementation increases digestibility and pancreatic secretion in young milk-fed calves. J. Dairy. Sci. 2010, 93, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- Lacorn, M.; Goerke, M.; Claus, R. Inulin-coated butyrate increases ileal MCT 1 expression and affects mucosal morphology in the porcine ileum by reduced apoptosis. J. Anim. Physiol. Anim. Nutr. 2010, 94, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Gunthner, D.; Letzguss, H. Effects of feeding fat-coated butyrate on mucosal morphology and function in the small intestine of the pig. J. Anim. Physiol. Anim. Nutr. 2007, 91, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br. J. Nutr. 2013, 110, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourcyrous, M.; Nolan, V.G.; Goodwin, A.; Davis, S.L.; Buddington, R.K. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Favre, A.; Szylit, O.; Popot, F.; Catala, I.; Rondeau, C.; Maurage, C.; Gold, F.; Borderon, J.C.; Butel, M.J. Diet, length of gestation, and fecal short chain fatty acids in healthy premature neonates. J. Parenter. Enteral. Nutr. 2002, 26, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [PubMed]
- Vital, M.; Gao, J.; Rizzo, M.; Harrison, T.; Tiedje, J.M. Diet is a major factor governing the fecal butyrate-producing community structure across Mammalia, Aves and Reptilia. ISME J. 2015, 9, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 5, 1–17. [Google Scholar] [CrossRef]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martinez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [PubMed]
- Gower, B.A.; Bergman, R.; Stefanovski, D.; Darnell, B.; Ovalle, F.; Fisher, G.; Sweatt, S.K.; Resuehr, H.S.; Pelkman, C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: A randomized, controlled trial. Nutr. Metab. 2016, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Klosterbuer, A.S.; Hullar, M.A.; Li, F.; Traylor, E.; Lampe, J.W.; Thomas, W.; Slavin, J.L. Gastrointestinal effects of resistant starch, soluble maize fibre and pullulan in healthy adults. Br. J. Nutr. 2013, 110, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Walter, J.; Ramer-Tait, A.E. Resistant starches for the management of metabolic diseases. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes. Rev. 2013, 14, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; Keshavarzian, A.; Patterson, J.A.; Venkatachalam, M.; Gillevet, P.; Hamaker, B.R. Starch-entrapped microspheres extend in vitro fecal fermentation, increase butyrate production, and influence microbiota pattern. Mol. Nutr. Food Res. 2009, 1, S121–S130. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Rose, D.J.; Rumpagaporn, P.; Patterson, J.A.; Hamaker, B.R. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J. Food Sci. 2011, 76, H137–H142. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; Venema, K.; Keshavarzian, A.; Hamaker, B.R. Starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br. J. Nutr. 2010, 103, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Grooms, K.N.; Ommerborn, M.J.; Pham, D.Q.; Djousse, L.; Clark, C.R. Dietary fiber intake and cardiometabolic risks among, U.S. adults, NHANES 1999–2010. Am. J. Med. 2013, 126, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Dodd, K.W.; Juan, W.Y.; Johnson, L.K.; Jahns, L. Whole Grains Contribute Only a Small Proportion of Dietary Fiber to the U.S. Diet. Nutrients 2017, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moens, F.; Weckx, S.; De Vuyst, L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int. J. Food Microbiol. 2016, 231, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Singh, N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol. 2013, 13, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Charney, A.N.; Micic, L.; Egnor, R.W. Nonionic diffusion of short-chain fatty acids across rat colon. Am. J. Physiol. 1998, 274, G518–G524. [Google Scholar] [PubMed]
- Cresci, G.A.; Thangaraju, M.; Mellinger, J.D.; Liu, K.; Ganapathy, V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR 109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 2010, 14, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Harig, J.M.; Ng, E.K.; Dudeja, P.K.; Brasitus, T.A.; Ramaswamy, K. Transport of n-butyrate into human colonic luminal membrane vesicles. Am. J. Physiol. 1996, 271, G415–G422. [Google Scholar] [PubMed]
- Ritzhaupt, A.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. The characterization of butyrate transport across pig and human colonic luminal membrane. J. Physiol. 1998, 507, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Krishnan, S.; Ramakrishna, B.S.; Mathan, M.; Pulimood, A.B.; Murthy, S.N. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut 2000, 46, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N.M. GPR43/FFA2, physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR 43 and GPR 109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, Z.G.; Dhanasekaran, D.N. G protein regulation of MAPK networks. Oncogene 2007, 26, 3122–3142. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR 41 and GPR 43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, W.N.; Douangpanya, J.; Mu, S.; Jaeckel, P.; Zhang, M.; Maxwell, J.R.; Rottman, J.B.; Labitzke, K.; Willee, A.; Beckmann, H.; et al. Differing roles for short chain fatty acids and GPR 43 agonism in the regulation of intestinal barrier function and immune responses. PLoS ONE 2017, 12, e0180190. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Plaisancié, P.; Dumoulin, V.; Chayvialle, J.-A.; Cuber, J.-C. Luminal peptide YY-releasing factors in the isolated vascularly perfused rat colon. J. Endocrinol. 1996, 151, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.K.; Haver, A.C.; Reidelberger, R.D. Intravenous Infusion of Peptide YY (3–36) Potently Inhibits Food Intake in Rats. Endocrinology 2005, 146, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Ficca, M.; Kirkland, J.B. Niacin. Adv. Nutr. Int. Rev. J. 2016, 7, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Chan, L.; Leung, P. Involvement of the Niacin Receptor GPR 109a in the LocalControl of Glucose Uptake in Small Intestine of Type 2 Diabetic Mice. Nutrients 2015, 7, 5352. [Google Scholar] [CrossRef] [PubMed]
- Steliou, K.; Boosalis, M.S.; Perrine, S.P.; Sangerman, J.; Faller, D.V. Butyrate Histone Deacetylase Inhibitors. Bio. Res. Open Access 2012, 1, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of Histone Deacetylase Activity by Butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.E. Reinventing cancer cell metabolism. Clin. Cancer Res. 2012, 18, 5536. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.C.; Olson, K.A.; Jiang, L.; Hawkins, A.J.; Van Vranken, J.G.; Xie, J.; Egnatchik, R.A.; Earl, E.G.; DeBerardinis, R.J.; Rutter, J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol. Cell 2014, 56, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. The microbiome and its potential as a cancer preventive intervention. Semin. Oncol. 2016, 43, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Encarnacao, J.C.; Abrantes, A.M.; Pires, A.S.; Botelho, M.F. Revisit dietary fiber on colorectal cancer: Butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015, 34, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA, 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.M.; Chomienne, O.; Gubler, M.; Arduini, A. Carnitine palmitoyltransferase (CPT) modulators: A medicinal chemistry perspective on 35 years of research. J. Med. Chem. 2011, 54, 3109–3152. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Ramachandran, S.; Ganapathy, V. Cell-Surface and Nuclear Receptors in the Colon as Targets for Bacterial Metabolites and Its Relevance to Colon Health. Nutrients 2017, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Bennett, N.; MacDonald, A.; Johnstone, M.; Whelan, J.; Donohoe, D.R. Cellular Metabolism and Dose Reveal Carnitine-Dependent and -Independent Mechanisms of Butyrate Oxidation in Colorectal Cancer Cells. J. Cell Physiol. 2016, 231, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Elimrani, I.; Lahjouji, K.; Seidman, E.; Roy, M.J.; Mitchell, G.A.; Qureshi, I. Expression and localization of organic cation/carnitine transporter OCT N2 in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G863–G871. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Myeroff, L.; Smiraglia, D.; Romero, M.F.; Pretlow, T.P.; Kasturi, L.; Lutterbaugh, J.; Rerko, R.M.; Casey, G.; Issa, J.P.; et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc. Natl. Acad. Sci. USA 2003, 100, 8412–8417. [Google Scholar] [CrossRef] [PubMed]
- Elimrani, I.; Dionne, S.; Saragosti, D.; Qureshi, I.; Levy, E.; Delvin, E.; Seidman, E.G. Acetylcarnitine potentiates the anticarcinogenic effects of butyrate on SW480 colon cancer cells. Int. J. Oncol. 2015, 47, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Moeinian, M.; Ghasemi-Niri, S.F.; Mozaffari, S.; Abdolghaffari, A.H.; Baeeri, M.; Navaea-Nigjeh, M.; Abdollahi, M. Beneficial effect of butyrate, Lactobacillus casei and L-carnitine combination in preference to each in experimental colitis. World J. Gastroenterol. 2014, 20, 10876–10885. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, H.J.; Zhang, Z.Q.; Chen, Q.; Liu, B.; Wu, J.P.; Zhu, L. L-carnitine ameliorates cancer cachexia in mice by regulating the expression and activity of carnitine palmityl transferase. Cancer Biol. Ther. 2011, 12, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, Z.; Zhang, Y.; Pan, X.; Yu, L.; Liu, S. L-Carnitine Ameliorates Cancer Cachexia in Mice Partly via the Carnitine Palmitoyltransferase-Associated PPA R-gamma Signaling Pathway. Oncol. Res. Treat. 2015, 38, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming growth factor-beta: A therapeutic target for cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulos, P.; Zizi-Sermpetzoglou, A.; Rizos, S.; Kostakis, A.; Nikiteas, N.; Papavassiliou, A.G. TGF-beta signalling in colon carcinogenesis. Cancer Lett. 2012, 314, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mithani, S.K.; Balch, G.C.; Shiou, S.R.; Whitehead, R.H.; Datta, P.K.; Beauchamp, R.D. Smad3 has a critical role in TGF-beta-mediated growth inhibition and apoptosis in colonic epithelial cells. J. Surg. Res. 2004, 117, 296–305. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, Z.; Ma, J.; Chen, J.; Peng, J.; Yang, S.; He, Y. Deregulation of miR-193b affects the growth of colon cancer cells via transforming growth factor-beta and regulation of the SMA D3 pathway. Oncol. Lett. 2017, 13, 2557–2562. [Google Scholar] [PubMed]
- Nguyen, K.A.; Cao, Y.; Chen, J.R.; Townsend, C.M., Jr.; Ko, T.C. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Ann. Surg. 2006, 243, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Gao, X.X.; Zhang, W.L.; Zhang, G.H.; Nguyen, A.K.; Liu, X.H.; Jimenez, F.; Cox, C.S.; Townsend, C.M.; Ko, T.C. Dietary fiber enhances TGF-beta signaling and growth inhibition in the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G156–G164. [Google Scholar] [CrossRef] [PubMed]
- Bordonaro, M.; Lazarova, D.L. CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer. World J. Gastroenterol. 2015, 21, 8238–8248. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, D.L.; Chiaro, C.; Wong, T.; Drago, E.; Rainey, A.; O’Malley, S.; Bordonaro, M. CBP Activity Mediates Effects of the Histone Deacetylase Inhibitor Butyrate on WNT Activity and Apoptosis in Colon Cancer Cells. J. Cancer 2013, 4, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, D.L.; Bordonaro, M.; Carbone, R.; Sartorelli, A.C. Linear relationship between Wnt activity levels and apoptosis in colorectal carcinoma cells exposed to butyrate. Int. J. Cancer 2004, 110, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Bordonaro, M.; Lazarova, D.L.; Sartorelli, A.C. Butyrate and Wnt signaling: A possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle 2008, 7, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, D.L.; Bordonaro, M. Vimentin, colon cancer progression and resistance to butyrate and other HDA Cis. J. Cell Mol. Med. 2016, 20, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Bordonaro, M.; Tewari, S.; Cicco, C.E.; Atamna, W.; Lazarova, D.L. A switch from canonical to noncanonical Wnt signaling mediates drug resistance in colon cancer cells. PLoS ONE 2011, 6, e27308. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-protein–Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [PubMed]
- Gibson, P.; Rosella, O. Interleukin 8 secretion by colonic crypt cells in vitro: Response to injury suppressed by butyrate and enhanced in inflammatory bowel disease. Gut 1995, 37, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar] [PubMed]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [PubMed]
- Cimini, F.A.; Barchetta, I.; Porzia, A.; Mainiero, F.; Costantino, C.; Bertoccini, L.; Ceccarelli, V.; Morini, S.; Baroni, M.G.; Lenzi, A.; et al. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta. Diabetol. 2017, 54, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, G.P.; Haddad, D.O.; Fagundes, V.O.; Vargas, B.K.; Kloecker, A.; Romao, P.R.; Peres, A. High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight-obese individuals. Cytokine 2016, 77, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Liu, J.; Gao, X.; Yang, H.; Chen, W.; Li, W.; Li, Y.; Yang, W.; Wang, B. Palmitate induces interleukin-8 expression in human aortic vascular smooth muscle cells via Toll-like receptor 4/nuclear factor-kappaB pathway (TLR4/NF-kappaB-8). J. Diabetes 2014, 6, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Walker, W.A.; Sanderson, I.R. Butyrate regulates the expression of pathogen-triggered IL-8 in intestinal epithelia. Pediatr. Res. 2007, 62, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Coornaert, B.; Carpentier, I.; Beyaert, R. A20, central gatekeeper in inflammation and immunity. J. Biol. Chem. 2009, 284, 8217–8221. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends. Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Bamba, T.; Sasaki, M. Physiological and anti-inflammatory roles of dietary fiber and butyrate in intestinal functions. JPEN J. Parenter. Enteral. Nutr. 1999, 23, S70–S73. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary fibre-based SCF A mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Winer, D.A.; Luck, H.; Tsai, S.; Winer, S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016, 23, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and Diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Baskin, M.L.; Ard, J.; Franklin, F.; Allison, D.B. Prevalence of obesity in the United States. Obes. Rev. 2005, 6, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.G.; Williamson, D.F.; Glick, H.A. Direct medical cost of overweight and obesity in the United States: A quantitative systematic review. Obes. Rev. 2011, 12, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.A.; Levine, R. The economic impact of obesity in the United States. Diabetes Metab. Syndr. Obes. 2010, 3, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Goettler, A.; Grosse, A.; Sonntag, D. Productivity loss due to overweight and obesity: A systematic review of indirect costs. BMJ Open 2017, 7, e014632. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, N.; Abouzaid, S.; Andersen, L.; Wang, Z.; Powers, A. Cohort analysis assessing medical and nonmedical cost associated with obesity in the workplace. J. Occup. Environ. Med. 2014, 56, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ahn, J.; Huang, W.Y.; Hayes, R.B. Association of obesity with cardiovascular disease mortality in the PLC O trial. Prev. Med. 2013, 57, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Matsushita, K.; Lazo, M.; Bello, N.; Blumenthal, R.S.; Gerstenblith, G.; Nambi, V.; Ballantyne, C.M.; Solomon, S.D.; Selvin, E.; et al. Obesity and Subtypes of Incident Cardiovascular Disease. J. Am. Heart Assoc. 2016, 5, e003921. [Google Scholar] [CrossRef] [PubMed]
- Hinnouho, G.M.; Czernichow, S.; Dugravot, A.; Nabi, H.; Brunner, E.J.; Kivimaki, M.; Singh-Manoux, A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort study. Eur. Heart J. 2015, 36, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Deepa, M.; Shivashankar, R.; Ali, M.K.; Kapoor, D.; Gupta, R.; Lall, D.; Tandon, N.; Mohan, V.; Kadir, M.M.; et al. Comparison of multiple obesity indices for cardiovascular disease risk classification in South Asian adults: The CAR RS Study. PLoS ONE 2017, 12, e0174251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Wang, J.; Yan, Z.; Luo, J. Excess body weight and the risk of primary liver cancer: An updated meta-analysis of prospective studies. Eur. J. Cancer 2012, 48, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Newton, C.C.; Freedman, N.D.; Koshiol, J.; Alavanja, M.C.; Beane Freeman, L.E.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Datta, M.; et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res. 2016, 76, 6076–6083. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS ONE 2013, 8, e53916. [Google Scholar] [CrossRef] [PubMed]
- Gathirua-Mwangi, W.G.; Monahan, P.; Song, Y.; Zollinger, T.W.; Champion, V.L.; Stump, T.E.; Imperiale, T.F. Changes in Adult BMI and Waist Circumference Are Associated with Increased Risk of Advanced Colorectal Neoplasia. Dig. Dis. Sci. 2017, 62, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Hou, D.; Zhao, X.; Liu, J.; Cheng, H.; Wang, Y.; Mi, J. Childhood Adiposity and Nonalcoholic Fatty Liver Disease in Adulthood. Pediatrics 2017, 139, e20162738. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Khan, S.S.; Ning, H.; Siddique, J.; Lewis, C.E.; Carr, J.J.; Vos, M.B.; Speliotes, E.; Terrault, N.A.; Rinella, M.E.; et al. Body Mass Index Trajectories in Young Adulthood Predict Nonalcoholic Fatty Liver Disease in Middle Age: The CARDIA Cohort Study. Liver Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.H.; Wagner, G.; Heitmann, B.L. Sexual function and obesity. Int. J. Obes. 2007, 31, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Rowland, D.L.; McNabney, S.M.; Mann, A.R. Sexual Function, Obesity, and Weight Loss in Men and Women. Sex Med. Rev. 2017, 5, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and type 2 diabetes: What can be unified and what needs to be individualized? J. Clin. Endocrinol. Metab. 2011, 96, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care 2012, 35, S11–S63. [Google Scholar]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Sun, J.; Chen, Y.Q. Clostridium Butyricum CGM CC0313.1 Modulates Lipid Profile, Insulin Resistance and Colon Homeostasis in Obese Mice. PLoS ONE 2016, 11, e0154373. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jena, G. Sodium butyrate reduces insulin-resistance, fat accumulation and dyslipidemia in type-2 diabetic rat: A comparative study with metformin. Chem. Biol. Interact. 2016, 254, 124–134. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Dong, L.; Xu, W.; Bai, K.; Lu, C.; Wu, Y.; Huang, Q.; Zhang, L.; Wang, T. Dietary Tributyrin Supplementation Attenuates Insulin Resistance and Abnormal Lipid Metabolism in Suckling Piglets with Intrauterine Growth Retardation. PLoS ONE 2015, 10, e0136848. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, F.; Guice, J.; Page, R.; Welsh, D.A.; Taylor, C.M.; Blanchard, E.E.; Luo, M.; Raggio, A.M.; Stout, R.W.; Carvajal-Aldaz, D.; et al. Obese ZDF rats fermented resistant starch with effects on gut microbiota but no reduction in abdominal fat. Mol. Nutr. Food Res. 2017, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Q.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. A comparative study of the antidiabetic effects exerted by live and dead multi-strain probiotics in the type 2 diabetes model of mice. Food Funct. 2016, 7, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R., 2nd; Boyle, K.E.; Koves, T.R.; Ilkayeva, O.R.; Muoio, D.M.; Houmard, J.A.; Friedman, J.E. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity (Silver Spring) 2015, 23, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.E.; Canham, J.P.; Consitt, L.A.; Zheng, D.; Koves, T.R.; Gavin, T.P.; Holbert, D.; Neufer, P.D.; Ilkayeva, O.; Muoio, D.M.; et al. A high-fat diet elicits differential responses in genes coordinating oxidative metabolism in skeletal muscle of lean and obese individuals. J. Clin. Endocrinol. Metab. 2011, 96, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Mai, M.; Tonjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, C.; Chen, L.; Han, X.; Ji, L. Human serum acylcarnitine profiles in different glucose tolerance states. Diabetes Res. Clin. Pract. 2014, 104, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.J.; Sellmann, C.; Engstler, A.J.; Ziegenhardt, D.; Bergheim, I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br. J. Nutr. 2015, 114, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Iacono, A.; Santoro, A.; Paciello, O.; Ferrante, M.C.; Canani, R.B.; Calignano, A.; Meli, R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE 2013, 8, e68626. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Zou, J.Y.; Hu, E.D.; Chen, D.Z.; Chen, L.; Lu, F.B.; Xu, L.M.; Zheng, M.H.; Li, H.; Huang, Y.; et al. Sodium butyrate ameliorates S100/FCA-induced autoimmune hepatitis through regulation of intestinal tight junction and toll-like receptor 4 signaling pathway. Immunol. Lett. 2017, 190, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, Y.; Zhang, H.; Wang, J.; Zhu, J.; Wang, Y.; Guo, Y.; Wang, G.; Xu, T.; Chu, M.; et al. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct. 2017, 8, 4042–4052. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.H. Four grams of glucose. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E11–E21. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Reed, M.A.; Consitt, L.A.; Martin, O.J.; Haynie, K.R.; Hulver, M.W.; Muoio, D.M.; Dohm, G.L. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: Effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J. Clin. Endocrinol. Metab. 2010, 95, 3400–3410. [Google Scholar] [CrossRef] [PubMed]
- Koonen, D.P.; Sung, M.M.; Kao, C.K.; Dolinsky, V.W.; Koves, T.R.; Ilkayeva, O.; Jacobs, R.L.; Vance, D.E.; Light, P.E.; Muoio, D.M.; et al. Alterations in skeletal muscle fatty acid handling predisposes middle-aged mice to diet-induced insulin resistance. Diabetes 2010, 59, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Devarshi, P.P.; McNabney, S.M.; Henagan, T.M. Skeletal Muscle Nucleo-Mitochondrial Crosstalk in Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2017, 18, 831. [Google Scholar] [CrossRef] [PubMed]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Putti, R.; Migliaccio, V.; Sica, R.; Lionetti, L. Skeletal Muscle Mitochondrial Bioenergetics and Morphology in High Fat Diet Induced Obesity and Insulin Resistance: Focus on Dietary Fat Source. Front. Physiol. 2015, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.T.; Cornwell, P.D.; Dai, X.; Caguyong, M.J.; Ulrich, R.G. Agonists of the peroxisome proliferator-activated receptor alpha induce a fiber-type-selective transcriptional response in rat skeletal muscle. Toxicol. Sci. 2006, 92, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M.; Way, J.M.; Tanner, C.J.; Winegar, D.A.; Kliewer, S.A.; Houmard, J.A.; Kraus, W.E.; Dohm, G.L. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 2002, 51, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.A.; Bell, J.A.; Koves, T.R.; Muoio, D.M.; Hulver, M.W.; Haynie, K.R.; Dohm, G.L.; Houmard, J.A. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes 2010, 59, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Rumberger, J.M.; Arch, J.R.S.; Green, A. Butyrate and other short-chain fatty acids increase the rate of lipolysis in 3T3-L1 adipocytes. Peer. J. 2014, 2, e611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2017. [Google Scholar] [CrossRef] [PubMed]
- Krief, S.; Fève, B.; Baude, B.; Zilberfarb, V.; Strosberg, A.D.; Pairault, J.; Emorine, L.J. Transcriptional modulation by n-butyric acid of beta 1-, beta 2-, and beta 3-adrenergic receptor balance in 3T3-F442A adipocytes. J. Biol. Chem. 1994, 269, 6664–6670. [Google Scholar] [PubMed]
- Ding, S.T.; Smith, E.O.; McNeel, R.L.; Mersmann, H.J. Modulation of porcine adipocyte beta-adrenergic receptors by hormones and butyrate. J. Anim. Sci. 2000, 78, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, G.; Peng, Y.; Zhong, W.; Wang, Y.; Zhang, B. Sodium butyrate alleviates adipocyte inflammation by inhibiting NLR P3 pathway. Sci. Rep. 2015, 5, 12676. [Google Scholar] [CrossRef] [PubMed]
- Toscani, A.; Soprano, D.R.; Soprano, K.J. Sodium butyrate in combination with insulin or dexamethasone can terminally differentiate actively proliferating Swiss 3T3 cells into adipocytes. J. Biol. Chem. 1990, 265, 5722–5730. [Google Scholar] [PubMed]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR. Proc. Natl. Acad. Sci. USA, 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv. Nutr. 2016, 7, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Ostman, E.M.; Knudsen, K.E.; Holst, J.J.; Bjorck, I.M. A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J. Nutr. 2010, 140, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.M.; Ismail, N.A.; Thomas, E.L.; Fitzpatrick, J.A.; Bell, J.D.; Swann, J.R.; Costabile, A.; Childs, C.E.; Pedersen, C.; Goldstone, A.P.; et al. The impact of oligofructose on stimulation of gut hormones, appetite regulation and adiposity. Obesity 2014, 22, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, D.; Goossens, G.; Hermes, G.; Neis, E.; van der Beek, C.; Most, J.; Holst, J.; Lenaerts, K.; Kootte, R.S.; Nieuwdorp, M.; et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016, 24, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Muller, J.G.; Boxberger, F.; Dusel, G.; Richter, F.; Bartram, H.-P.; Christl, S.U.; Dempfle, C.-E.; Kasper, H. Histological changes in the colonic mucosa following irrigation with short-chain fatty acids. Eur. J. Gastroenterol. Hepatol. 1997, 9, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A Mixture of trans-Galactooligosaccharides Reduces Markers of Metabolic Syndrome and Modulates the Fecal Microbiota and Immune Function of Overweight Adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Schrade, K.B.; Vogt, J.A.; Tsihlias, E.B.; McBurney, M.I. Do colonic short-chain fatty acids contribute to the long-term adaptation of blood lipids in subjects with type 2 diabetes consuming a high-fiber diet? Am. J. Clin. Nutr. 2002, 75, 1023–1030. [Google Scholar] [PubMed]

| Acute Study | Short Chain Fatty Acid(s) or Dietary Fiber Composition | Control Group | Route of Administration | Duration of Treatment | Findings in Treatment Group Relative to the Control Group | Findings in Treatment Group Relative to Themselves (Longitudinal) |
| Nilsson et al., (2008) [199] | White wheat flour bread supplemented with barley fiber or resistant starch cereal-based meals (50 g available starch) | Unsupplemented white wheat flour bread (50 g available starch) | Oral—dietary supplement | 2 meals | ↑ glucose tolerance following a meal, GLP-1 and satiety; ↓ serum free fatty acids in men and women | |
| Vrieze et al., (2012) [204] | Microbial transplantation from lean donors | Microbial transplantation from own collected feces | Bowel lavage | Single intervention—measurements made 6 weeks after infusion | ↑ peripheral insulin sensitivity; no effect on resting energy expenditure in obese males with metabolic syndrome | |
| Chronic Study | Short Chain Fatty Acid(s) or Dietary Fiber Composition | Control Group | Route of Administration | Duration of Treatment | Findings in Treatment Group relative to the Control Group | Findings in Treatment Group Relative to Themselves (Longitudinal) |
| Dewulf et al., (2013) [205] | Oligofructose (16 g/day) | Dextrin maltose (16 g/day) | Oral—dietary supplement | 2 weeks | ↓ postprandial glucose AUC; ↑ PYY and GLP-1 in healthy men and women | |
| Gower et al., (2016) [49] | Resistant starch (30 g/day) | Waxy Corn Starch matched based on digestible starch in treatment group | Oral—dietary supplement | 4 weeks | ↑ insulin sensitivity in insulin resistant women; no effect in insulin sensitive women | |
| Parnell et al., (2009) [198] | Oligofructose (21 g/day) | Maltodextrin (21 g/day) | Oral—dietary supplement | 12 weeks | ↓ body weight, energy intake, fat mass and trunk fat, serum glucose and insulin and active ghrelin; ↑ PYY; no effect on GLP-1 in overweight/obese men and women | |
| Pedersen et al., (2013) [206] | Oligofructose (15–55 g/day) | none | Oral—dietary supplement | 5 weeks | ↑ PYY and satiety; no effects on energy intake, glucose, insulin, or GLP-1 in men and women | |
| Robertson et al., (2005) [48] | Resistant starch (30 g/day) + 20 g/day rapidly digestible starch | Rapidly digestible starch (Amioca; 20 g/day) | Oral—dietary supplement | 4 weeks | ↑ insulin sensitivity during meal tolerance test; ↑ glucose uptake by adipose tissue | |
| Scheppach et al., (1997) [207] | Sodium butyrate enema (100 mmol/L) or SCFA mixture (butyrate = 40 mmol/L | isotonic saline | Rectal | 8 weeks | ↓ polymorphonuclear leukocytes in lamina propria; ↓ upper intestinal crypt proliferation in individuals with active distal ulcerative colitis | |
| acetate = 60 mmol/L | (twice daily) | |||||
| propionate = 30 mmol/L) | ||||||
| Vulevic et al., (2013) [208] | Galactooligosaccharide (5.5 g/day) | Maltodextrin (5.5 g/day) | Oral—dietary supplement | 12 weeks | ↓ fasting insulin, triglycerides, total cholesterol and C-reactive protein in overweight/obese men and women | |
| Wolever et al., (2002) [209] | High fiber cereal (10–15% energy) | Low fiber cereal (10–15% energy) | Oral | 6 months | No effect on body weight, serum triacylglycerols or total cholesterol; nonsignificant ↓ HDL cholesterol in type 2 diabetic men and women |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. https://doi.org/10.3390/nu9121348
McNabney SM, Henagan TM. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients. 2017; 9(12):1348. https://doi.org/10.3390/nu9121348
Chicago/Turabian StyleMcNabney, Sean M., and Tara M. Henagan. 2017. "Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance" Nutrients 9, no. 12: 1348. https://doi.org/10.3390/nu9121348
APA StyleMcNabney, S. M., & Henagan, T. M. (2017). Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients, 9(12), 1348. https://doi.org/10.3390/nu9121348





