A New Numerical Scheme for Time Fractional Diffusive SEAIR Model with Non-Linear Incidence Rate: An Application to Computational Biology
Abstract
:1. Introduction
Preliminaries
2. Proposed Fractional Scheme
2.1. Stability of Proposed Fractional Scheme
2.2. Consistency of Fractional Proposed Scheme
2.3. Issue of Accuracy in Fractional NSFD
2.4. Issue of Consistency in Fractional NSFD
2.5. Convergence of Proposed Fractional Scheme
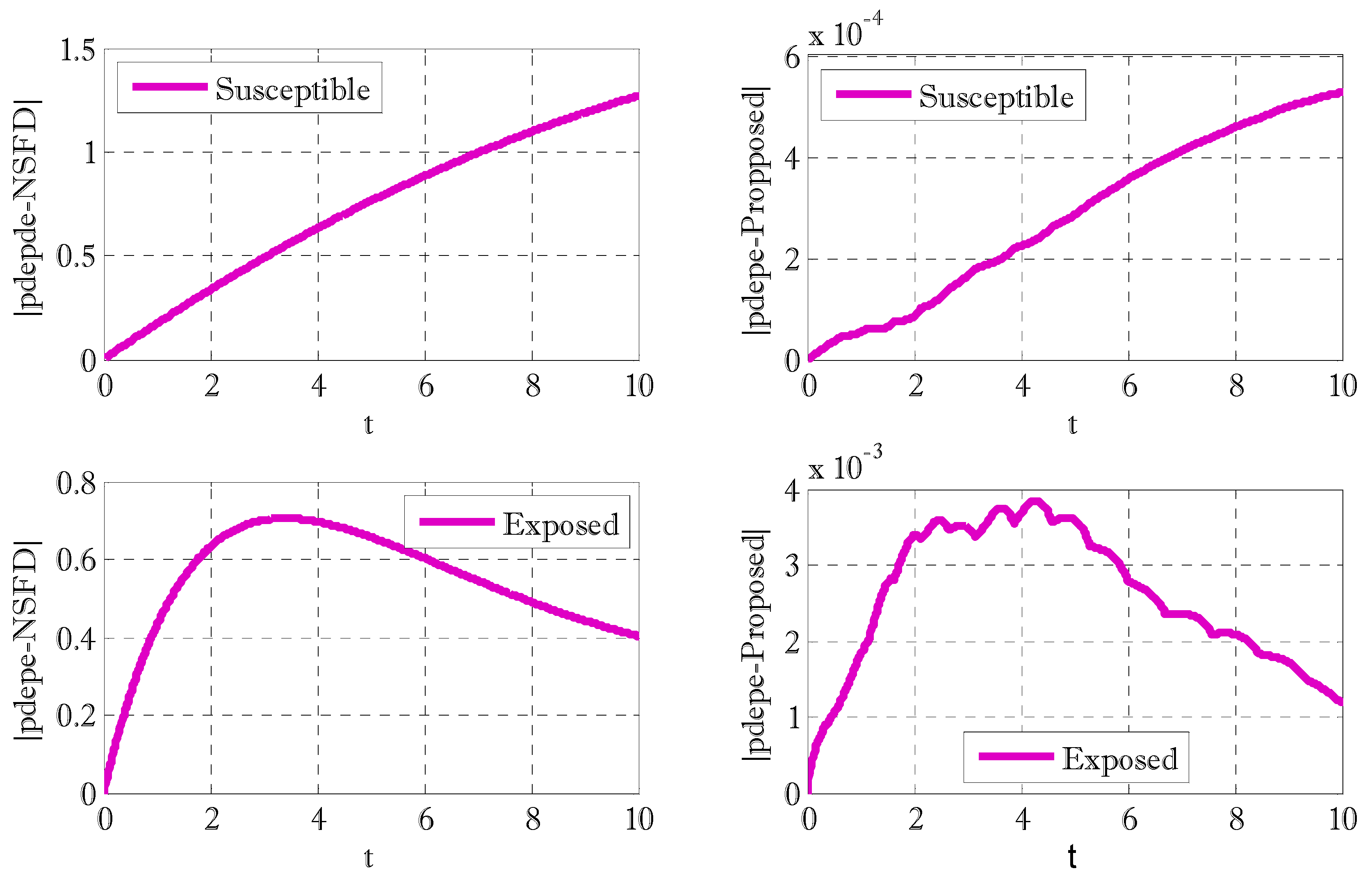
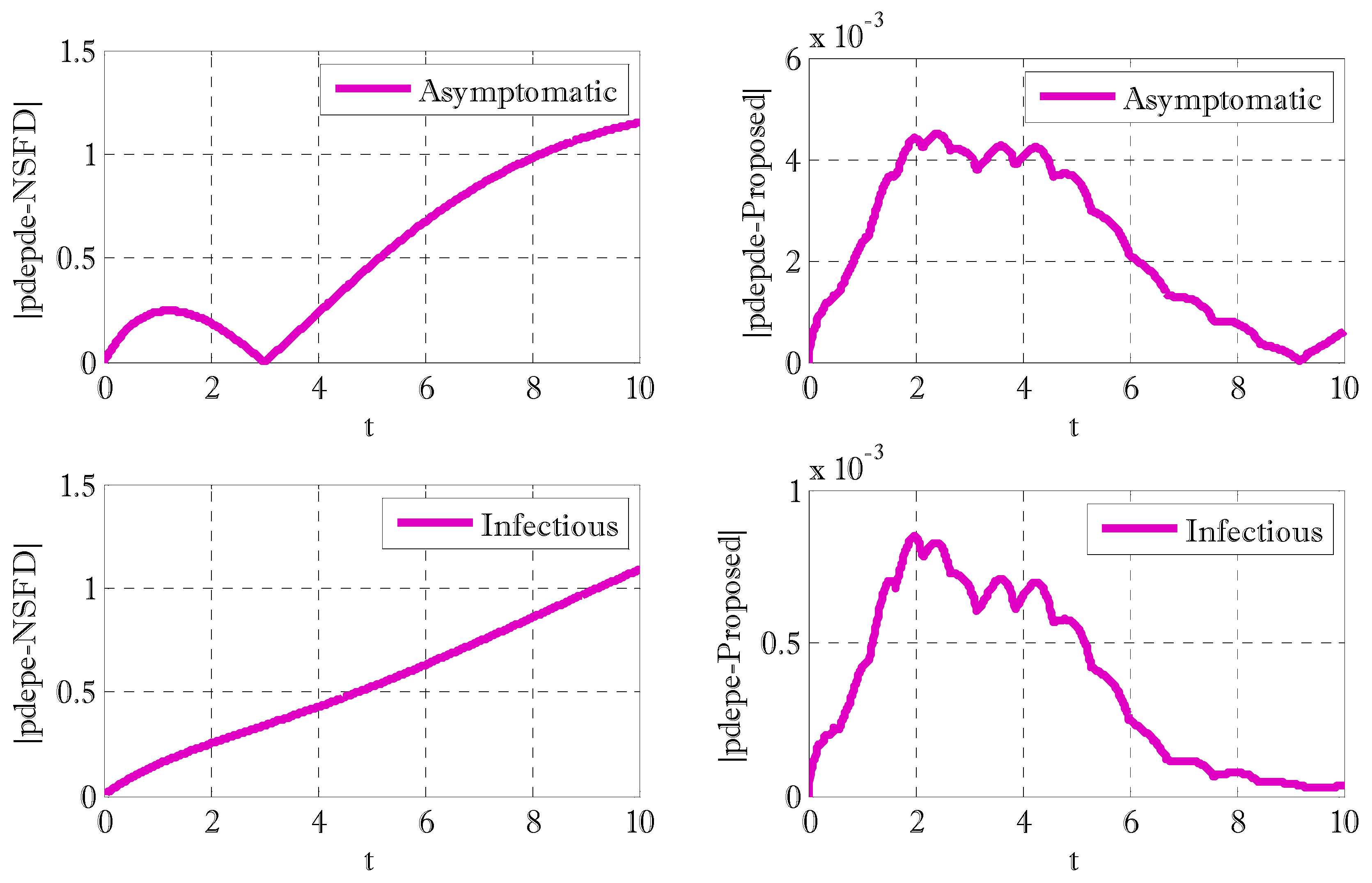
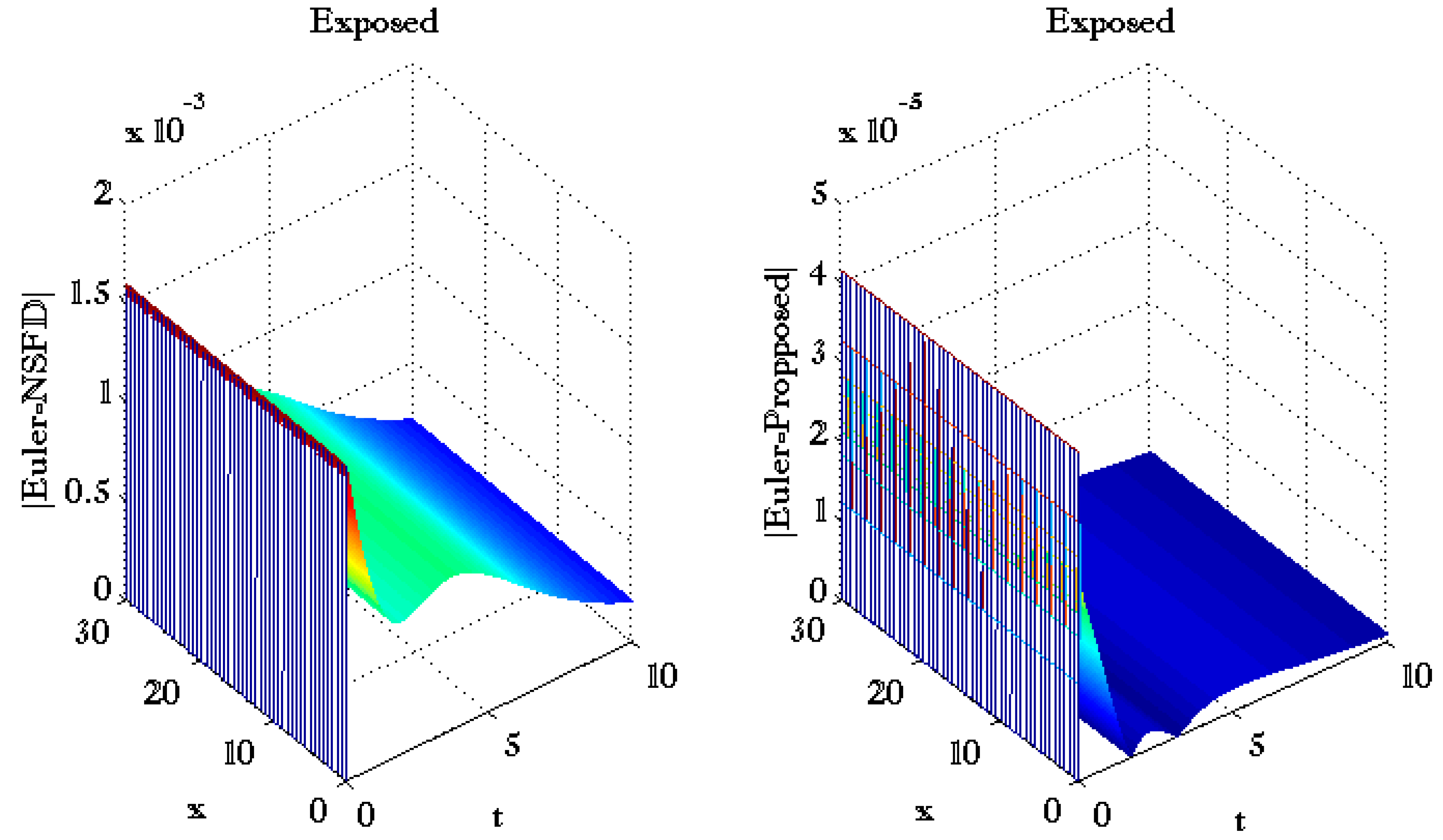
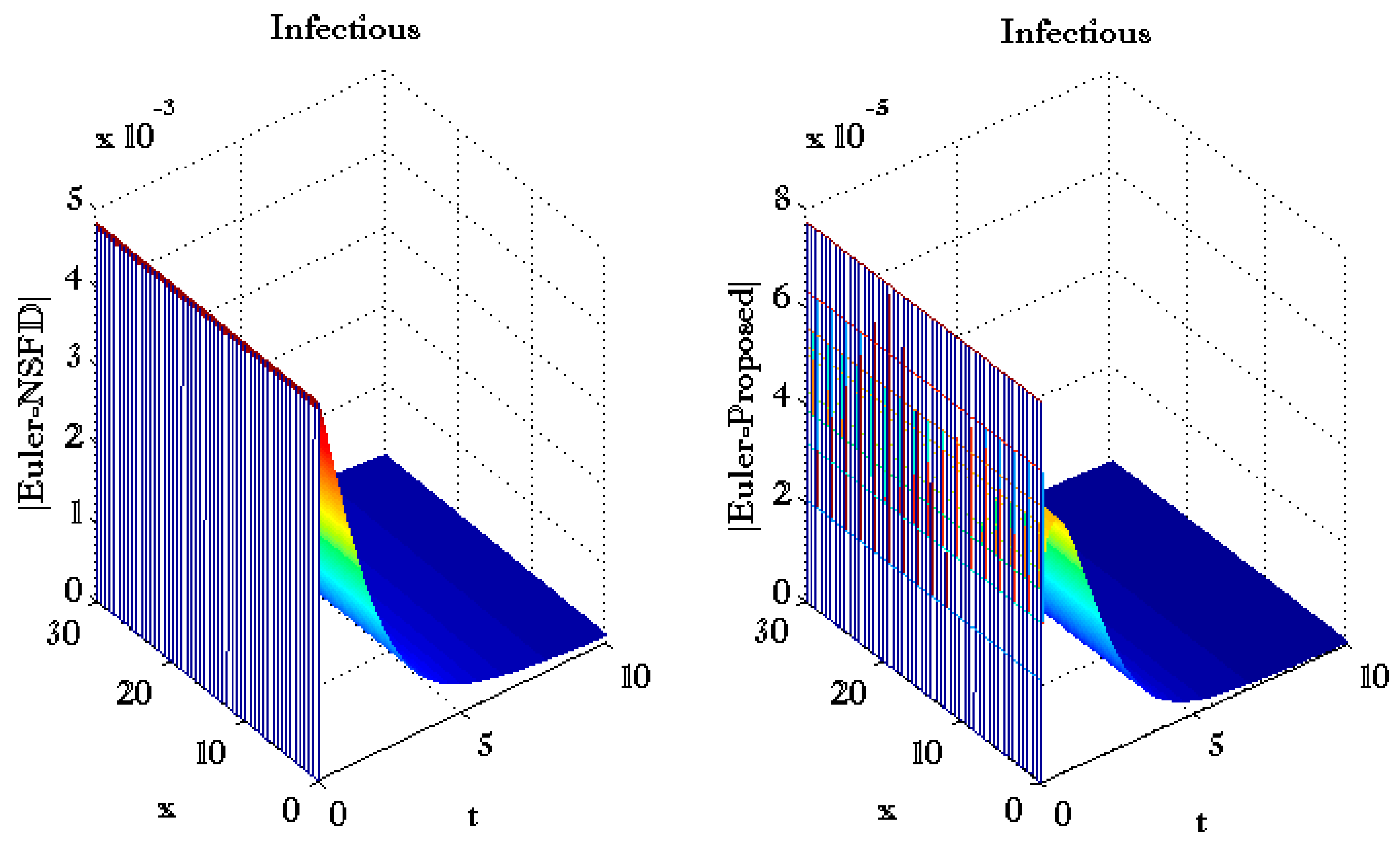
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). 2019. Available online: https://www.who.int/ (accessed on 29 April 2021).
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1927, 115, 700–721. [Google Scholar] [CrossRef] [Green Version]
- Hethcote, H.W. The Mathematics of Infectious Diseases. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef] [Green Version]
- Brauer, F. Mathematical epidemiology: Past, present, and future. Infect. Dis. Model. 2017, 2, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Atangana, A.; Alkahtani, B.S.T. Analysis of the Keller–Segel Model with a Fractional Derivative without Singular Kernel. Entropy 2015, 17, 4439–4453. [Google Scholar] [CrossRef]
- Thabet, S.T.; Abdo, M.S.; Shah, K.; Abdeljawad, T. Study of transmission dynamics of COVID-19 mathematical model under ABC fractional order derivative. Results Phys. 2020, 19, 103507. [Google Scholar] [CrossRef]
- Atangana, A.; Baleanu, D. New fractional derivatives with nonlocal and non-singular kernel: Theory and application to heat transfer model. Therm. Sci. 2016, 20, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Atangana, A.; Koca, I. On the new fractional derivative and application to nonlinear Baggs and Freedman model. J. Nonlinear Sci. Appl. 2016, 9, 2467–2480. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Atangana, A. Modeling the dynamics of novel coronavirus (2019-nCov) with fractional derivative. Alex. Eng. J. 2020, 59, 2379–2389. [Google Scholar] [CrossRef]
- Ullah, M.Z.; Alzahrani, A.K.; Baleanu, D. An efficient numerical technique for a new fractional tuberculosis model with nonsingular derivative operator. J. Taibah Univ. Sci. 2019, 13, 1147–1157. [Google Scholar] [CrossRef]
- Oluyori, D.A.; Adebayo, H.O.; Pérez, A.G.C. Global analysis of an SEIRS model for COVID-19 capturing saturated incidence with treatment response. Appl. Appl. Math. 2021, 16, 924–925. [Google Scholar]
- Okhuese, A.V. Estimation of the probability of reinfection with COVID-19 by the susceptible-exposed infectious-removed-undetectable-susceptible model. JMIR Public Health Surveill. 2020, 6, e19097. [Google Scholar] [CrossRef]
- Okhuese, A.V. Mathematical predictions for COVID-19 as a global pandemic. SSRN. 2020, p. 3555879, Preprint. Available online: https://ssrn.com/abstract=3555879 (accessed on 15 December 2021).
- Malkov, E. Simulation of coronavirus disease 2019 (COVID-19) scenarios with ossibility of reinfection. Chaos Solitons Fractals 2020, 139, 110296. [Google Scholar] [CrossRef] [PubMed]
- Mohd, M.H.; Sulayman, F. Unravelling the myths of R0 in controlling the dynamics of COVID-19 outbreak: A modelling perspective. Chaos Solitons Fractals 2020, 138, 109943. [Google Scholar] [CrossRef] [PubMed]
- Fichet-Calvet, E.; Rogers, D.J. Risk Maps of Lassa Fever in West Africa. PLOS Negl. Trop. Dis. 2009, 3, e388. [Google Scholar] [CrossRef]
- Bausch, D.G.; Schwarz, L. Outbreak of Ebola Virus Disease in Guinea: Where Ecology Meets Economy. PLoS Negl. Trop. Dis. 2014, 8, e3056. [Google Scholar] [CrossRef] [PubMed]
- Gumel, A.B.; McCluskey, C.C.; Watmough, J. An SVEIR model for assessing potential impact of an imperfectanti-SARS vaccine. Math. Biosci. Eng. 2006, 3, 485–512. [Google Scholar] [PubMed]
- Liu, X.; Takeuchi, Y.; Iwami, S. SVIR epidemic models with vaccination strategies. J. Theor. Biol. 2008, 253, 1–11. [Google Scholar] [CrossRef]
- Wei, H.; Jiang, Y.; Song, X.; Su, G.; Qiu, S. Global attractivity and permanence of a SVEIR epidemic model with pulse vaccination and time delay. J. Comput. Appl. Math. 2009, 229, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.-M.; Li, Z.; Song, X. Global analysis of an epidemic model with vaccination. J. Appl. Math. Comput. 2018, 57, 605–628. [Google Scholar] [CrossRef]
- Alexander, M.E.; Bowman, C.; Moghadas, S.M.; Summers, R.; Gumel, A.B.; Sahai, B.M. A vaccination model for transmission dynamics of influenza. SIAM J. Appl. Dyn. Syst. 2004, 3, 503–524. [Google Scholar] [CrossRef]
- Arino, J.; McCluskey, C.C.; Driessche, P.V.D. Global Results for an Epidemic Model with Vaccination that Exhibits Backward Bifurcation. SIAM J. Appl. Math. 2003, 64, 260–276. [Google Scholar] [CrossRef] [Green Version]
- Aldila, D. Cost-effectiveness and backward bifurcation analysis on COVID-19 transmission model considering direct and indirect transmission. Commun. Math. Biol. Neurosci. 2020, 2020, 49. [Google Scholar] [CrossRef]
- Aldila, D.; Khoshnaw, S.H.A.; Safitri, E.; Anwar, Y.R.; Bakry, A.R.Q.; Samiadji, B.M.; Anugerah, D.A.; Alfarizi, M.F.; Ayulani, I.D.; Salim, S.N. A mathematical study on the spread of COVID-19 considering social distancing and rapid assessment: The case of Jakarta, Indonesia. Chaos Solitons Fractals 2020, 139, 110042. [Google Scholar] [CrossRef] [PubMed]
- Daşbaşı, B. Stability Analysis of an Incommensurate Fractional-Order SIR Model. Math. Model. Numer. Simul. Appl. 2021, 1, 44–55. [Google Scholar] [CrossRef]
- Özköse, F.; Şenel, M.T.; Habbireeh, R. Fractional-order mathematical modelling of cancer cells-cancer stem cells-immune system interaction with chemotherapy. Math. Model. Numer. Simul. Appl. 2021, 1, 67–83. [Google Scholar] [CrossRef]
- Ikram, R.; Khan, A.; Zahri, M.; Saeed, A.; Yavuz, M.; Kumam, P. Extinction and stationary distribution of a stochastic COVID-19 epidemic model with time-delay. Comput. Biol. Med. 2021, 141, 105115. [Google Scholar] [CrossRef] [PubMed]
- Vahid RezaHosseini, W.-Z. The numerical solution of high dimensional variable-order time fractional diffusion equation via the singular boundary method. J. Adv. Res. 2021, 32, 73–84. [Google Scholar]
- Nikan, O.; Avazzadeh, Z. An improved localized radial basis-pseudospectral method for solving fractional reaction–subdiffusion problem. Results Phys. 2021, 23, 104048. [Google Scholar] [CrossRef]
- Aghdam, Y.E.; Khoshkhahtinat, M. High-accuracy numerical scheme for solving the space-time fractional advection-diffusion equation with convergence analysis. Alex. Eng. J. 2022, 61, 217–225. [Google Scholar] [CrossRef]
- Podlubny, I. Fractional Differential Equations; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Usero, D. Fractional Taylor Series for Caputo Fractional Derivatives, Construction of Numerical Schemes, Preprint. Available online: http://www.fdi.ucm.es/profesor/vazquez (accessed on 10 November 2021).
- Nawaz, Y.; Arif, M.S.; Ashraf, M.U. Development of Explicit Schemes for Diffusive SEAIR COVID-19 Epidemic Spreading Model: An Application to Computational Biology. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 2109–2119. [Google Scholar] [CrossRef]
- Nawaz, Y.; Arif, M.S.; Bibi, M.; Abbasi, J.N.; Javed, U.; Nazeer, A. A finite difference method and effective modification of gradient descent optimization algorithm for MHD fluid flow over a linearly stretching surface. Comput. Mater. Contin. 2020, 62, 657–677. [Google Scholar] [CrossRef]
- Nawaz, Y.; Arif, M.; Shatanawi, W.; Nazeer, A. An Explicit Fourth-Order Compact Numerical Scheme for Heat Transfer of Boundary Layer Flow. Energies 2021, 14, 3396. [Google Scholar] [CrossRef]



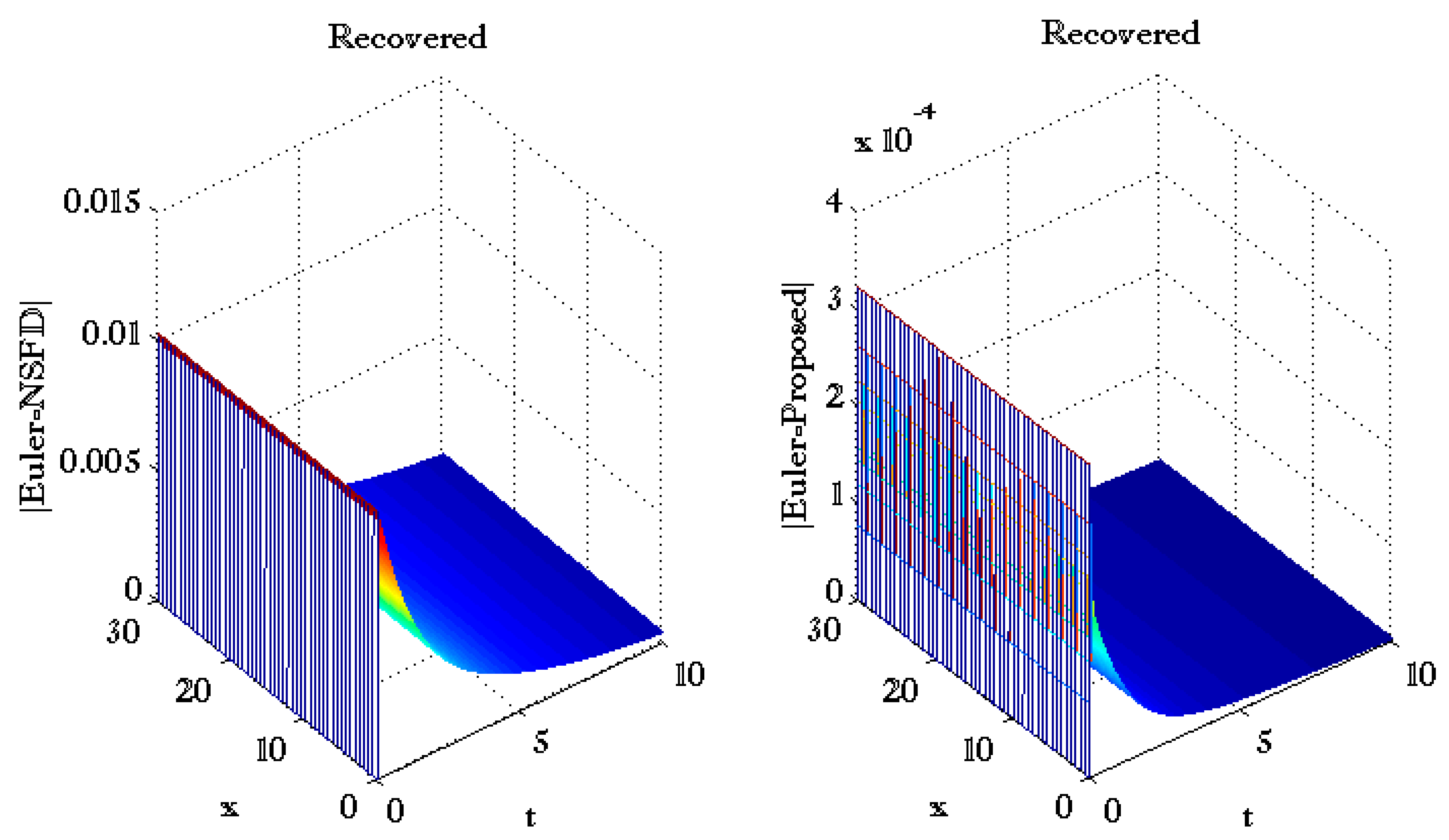
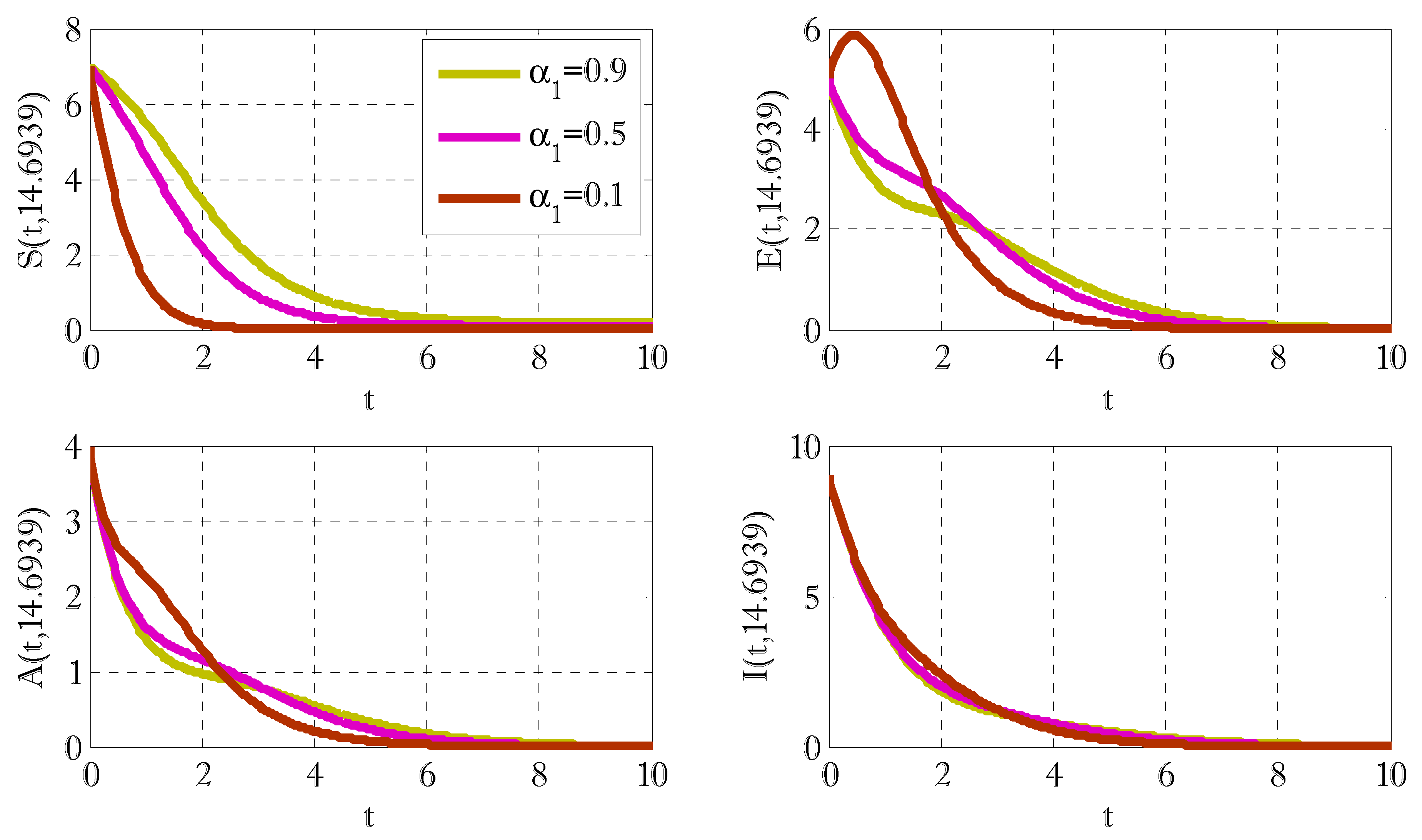
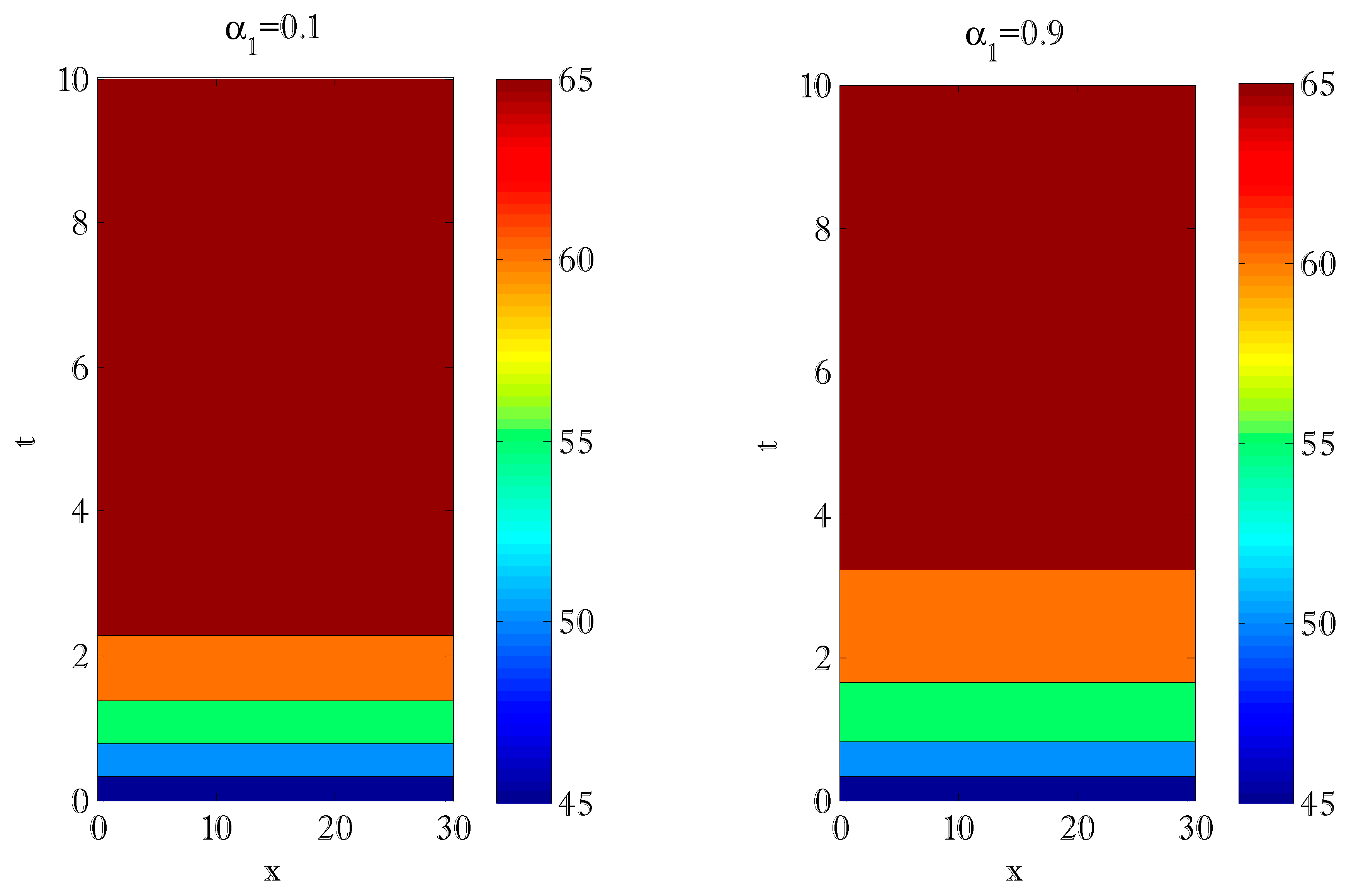


| Norm of Error | ||||||
| Proposed | NSFD | |||||
| 0.1 | 0.1 | 0.3 | 0.4 | 0.5 | 7.3001 × 10−4 | 3.3566 |
| 0.9 | 0.0060 | 4.3839 | ||||
| 0.9 | 0.0128 | 3.8177 | ||||
| 0.7 | 0.0147 | 4.3007 | ||||
| 0.9 | 0.0204 | 5.5968 | ||||
| 0.9 | 0.0099 | 1.7447 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, Y.; Arif, M.S.; Shatanawi, W. A New Numerical Scheme for Time Fractional Diffusive SEAIR Model with Non-Linear Incidence Rate: An Application to Computational Biology. Fractal Fract. 2022, 6, 78. https://doi.org/10.3390/fractalfract6020078
Nawaz Y, Arif MS, Shatanawi W. A New Numerical Scheme for Time Fractional Diffusive SEAIR Model with Non-Linear Incidence Rate: An Application to Computational Biology. Fractal and Fractional. 2022; 6(2):78. https://doi.org/10.3390/fractalfract6020078
Chicago/Turabian StyleNawaz, Yasir, Muhammad Shoaib Arif, and Wasfi Shatanawi. 2022. "A New Numerical Scheme for Time Fractional Diffusive SEAIR Model with Non-Linear Incidence Rate: An Application to Computational Biology" Fractal and Fractional 6, no. 2: 78. https://doi.org/10.3390/fractalfract6020078
APA StyleNawaz, Y., Arif, M. S., & Shatanawi, W. (2022). A New Numerical Scheme for Time Fractional Diffusive SEAIR Model with Non-Linear Incidence Rate: An Application to Computational Biology. Fractal and Fractional, 6(2), 78. https://doi.org/10.3390/fractalfract6020078








