Iodine-Enriched Urea Reduces Volatilization and Improves Nitrogen Uptake in Maize Plants
Abstract
:1. Introduction
2. Materials and Methods
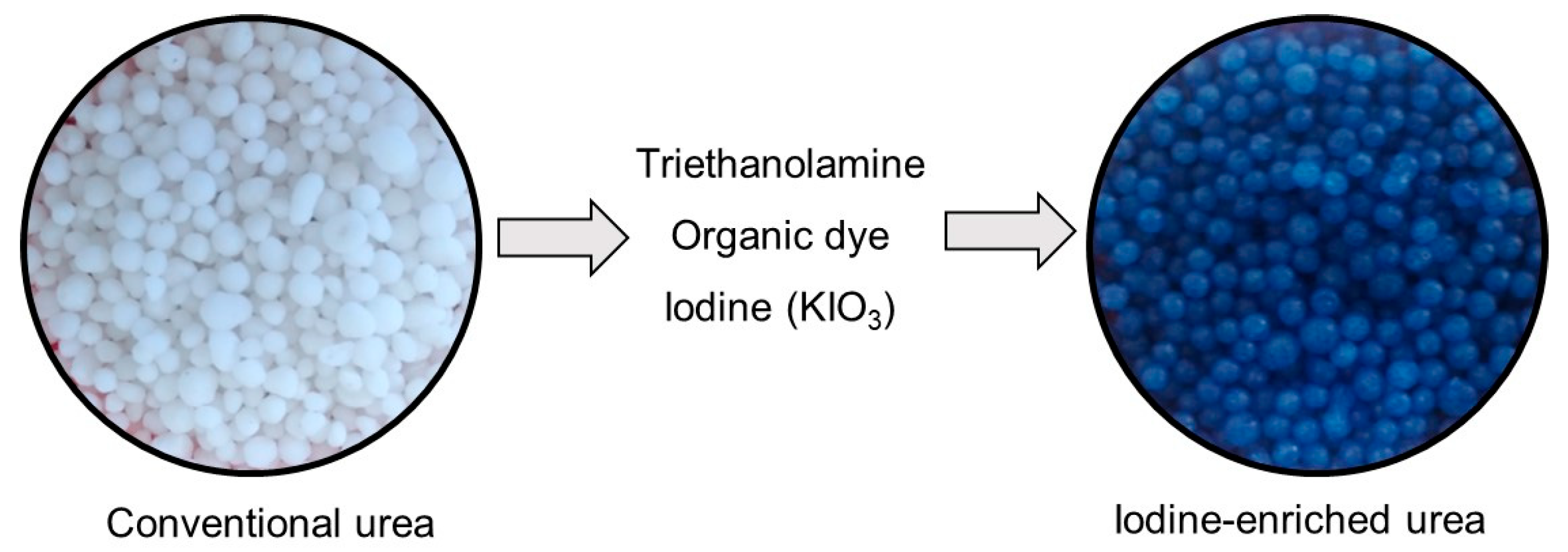
2.1. Synthesis of Iodine-Enriched Urea
2.2. Maize Growing and Treatments
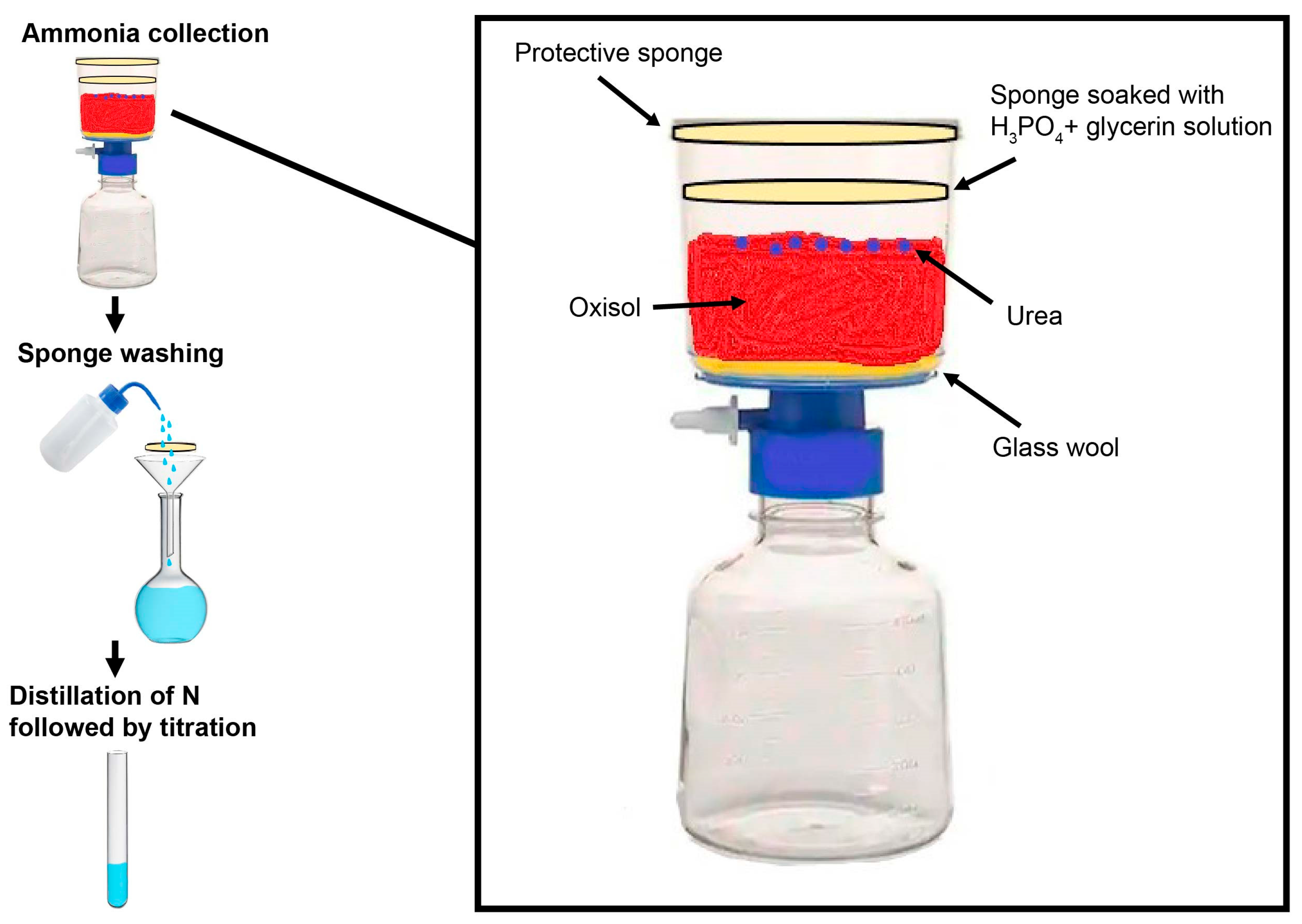
2.3. Nitrogen Volatilization
2.4. Statistical Analysis
3. Results
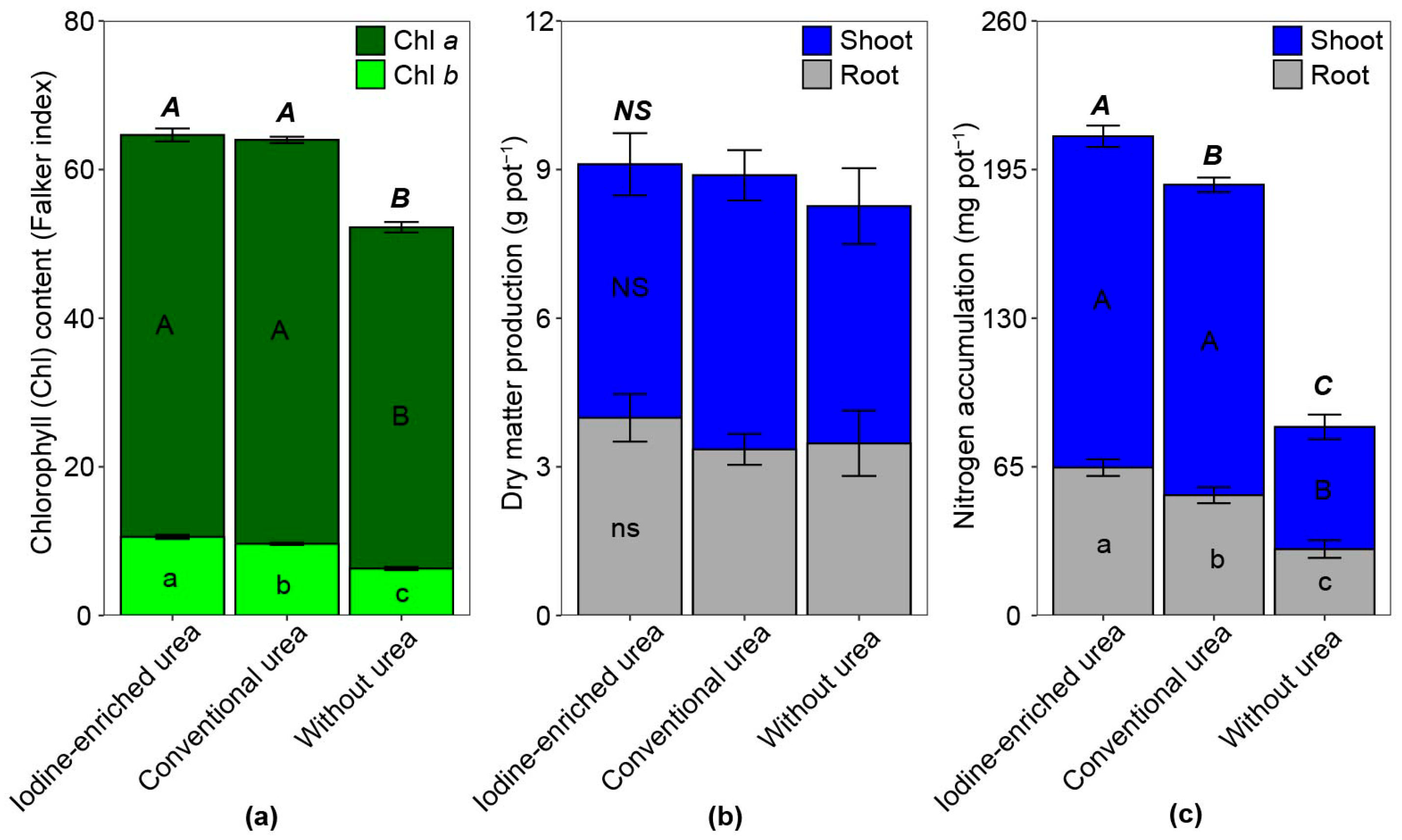
3.1. Maize Cultivation
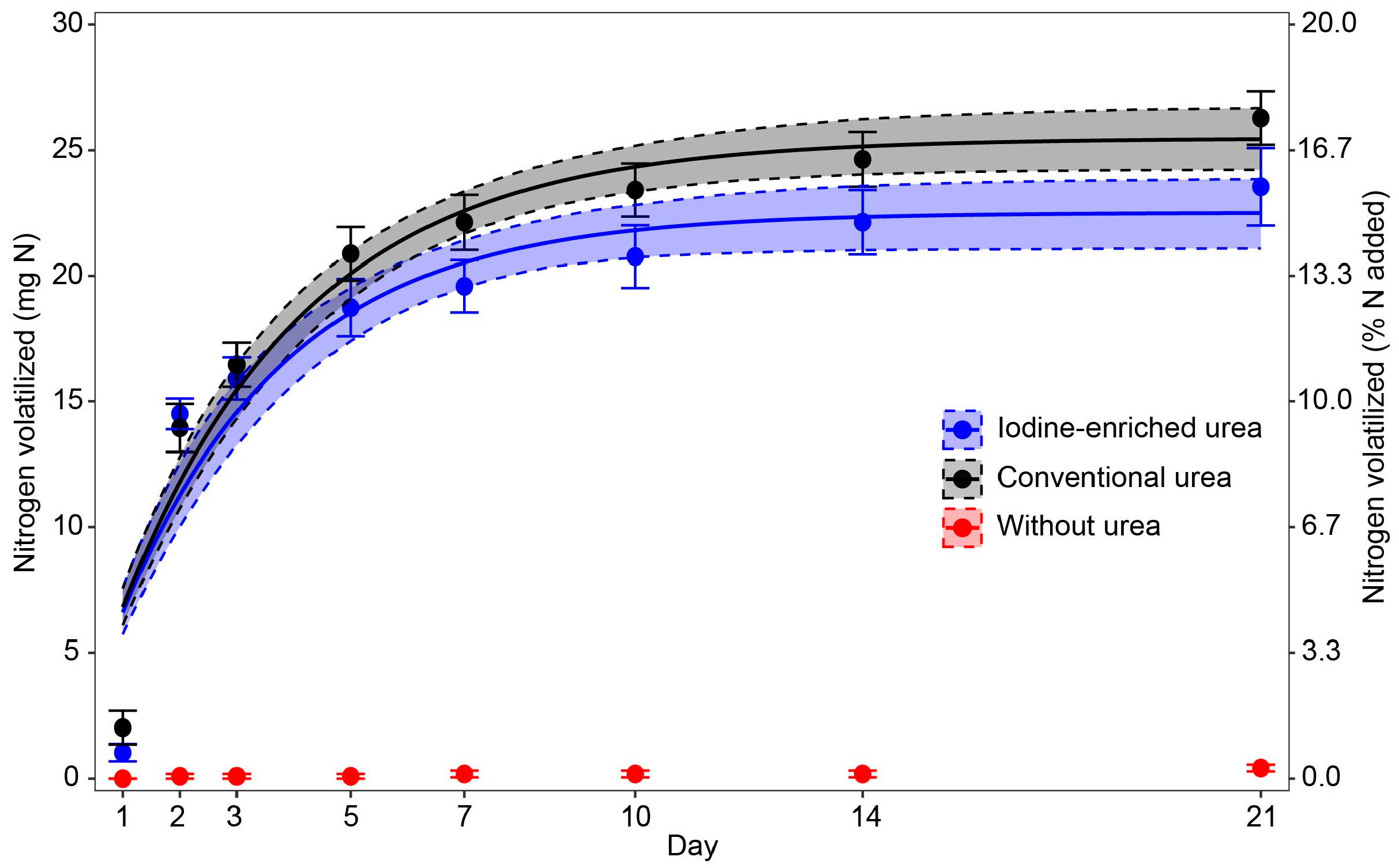
3.2. Nitrogen Volatilization
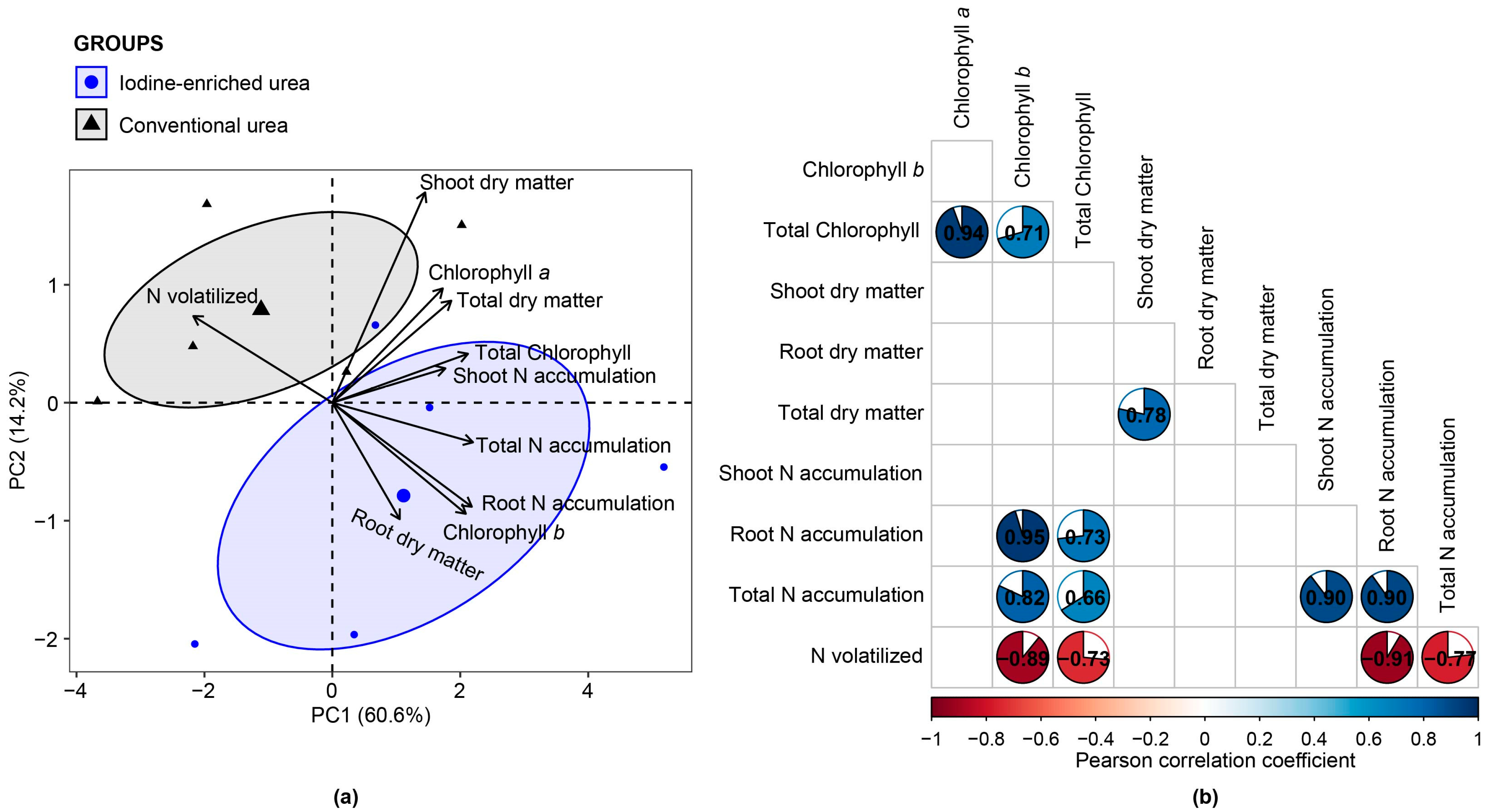
3.3. Multivariate and Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassim, B.M.A.R.; Lisboa, I.P.; Besen, M.R.; Otto, R.; Cantarella, H.; Inoue, T.T.; Batista, M.A. Nitrogen: From discovery, plant assimilation, sustainable usage to current enhanced efficiency fertilizers technologies—A review. Rev. Bras. Ciênc. Solo 2024, 48, e0230037. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations Fertilizer Production by Nutrient Type, World, 1961 to 2021. Available online: https://ourworldindata.org/grapher/fertilizer-production-by-nutrient-type-npk (accessed on 26 June 2024).
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: A review. Pedosphere 2023, 33, 385–406. [Google Scholar] [CrossRef]
- Harty, M.A.; McDonnell, K.P.; Whetton, R.; Gillespie, G.; Burke, J.I. Comparison of ammonia-N volatilization losses from untreated granular urea and granular urea treated with NutriSphere-N®. Soil Use Manag. 2024, 40, e12891. [Google Scholar] [CrossRef]
- Awale, R.; Chatterjee, A. Enhanced Efficiency Nitrogen Products Influence Ammonia Volatilization and Nitrous Oxide Emission from Two Contrasting Soils. Agron. J. 2017, 109, 47–57. [Google Scholar] [CrossRef]
- Gorh, D.; Baruah, K.K. Estimation of methane and nitrous oxide emission from wetland rice paddies with reference to global warming potential. Environ. Sci. Pollut. Res. 2019, 26, 16331–16344. [Google Scholar] [CrossRef]
- Pan, B.; Lam, S.K.; Mosier, A.; Luo, Y.; Chen, D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agric. Ecosyst. Environ. 2016, 232, 283–289. [Google Scholar] [CrossRef]
- Götze, H.; Saul, M.; Jiang, Y.; Pacholski, A. Effect of Incorporation Techniques and Soil Properties on NH3 and N2O Emissions after Urea Application. Agronomy 2023, 13, 2632. [Google Scholar] [CrossRef]
- Hube, S.; Alfaro, M.A.; Scheer, C.; Brunk, C.; Ramírez, L.; Rowlings, D.; Grace, P. Effect of nitrification and urease inhibitors on nitrous oxide and methane emissions from an oat crop in a volcanic ash soil. Agric. Ecosyst. Environ. 2017, 238, 46–54. [Google Scholar] [CrossRef]
- Yang, W.; Peng, Z.; Wang, G. An overview: Metal-based inhibitors of urease. J. Enzym. Inhib. Med. Chem. 2023, 38, 361–375. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; de Brito Silva, A.G. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Talma, M. Recent advances in design of new urease inhibitors: A review. J. Adv. Res. 2018, 13, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Chauhan, S.; Sangwan, S.; Kumar, R. Deepak Recent Advances in Anti-Urease Activity of Schiff Bases and their Metal Complexes. Asian J. Chem. 2024, 36, 779–794. [Google Scholar] [CrossRef]
- Pereira, L.E.T.; Herling, V.R.; Tech, A.R.B. Current Scenario and Perspectives for Nitrogen Fertilization Strategies on Tropical Perennial Grass Pastures: A Review. Agronomy 2022, 12, 2079. [Google Scholar] [CrossRef]
- Pursell, T.; Shirley, A.R., Jr.; Cochran, K.D.; Miller, J.M.; Holt, T.G.; Peeden, G.S. Urea Fertilizer Containing Central Volatilization Inhibitor Particles to Reduce Release of Ammonia and Processes for Making Same. U.S. Patent 8,758,474, 24 June 2014. [Google Scholar]
- Hellerman, L.; Perkins, M.E.; Clark, W.M. Urease Activity as Influenced by Oxidation and Reduction. Proc. Natl. Acad. Sci. USA 1933, 19, 855–860. [Google Scholar] [CrossRef]
- Smythe, C.V. The reaction of iodoacetate and of iodoacetamide with various sulfhydryl groups, with urease, and with yeast preparations. J. Biol. Chem. 1936, 114, 601–612. [Google Scholar] [CrossRef]
- Sindi, A.M.; Zaman, U.; Saleh, E.A.M.; Kassem, A.F.; Rahman, K.U.; Khan, S.U.; Alharbi, M.; Rizg, W.Y.; Omar, K.M.; Majrashi, M.A.A.; et al. Biochemical and thermodynamic properties of de novo synthesized urease from Vicia sativa seeds with enhanced industrial applications. Int. J. Biol. Macromol. 2024, 259, 129190. [Google Scholar] [CrossRef]
- Freitas, T.; Bartelega, L.; Santos, C.; Dutra, M.P.; Sarkis, L.F.; Guimarães, R.J.; Dominghetti, A.W.; Zito, P.C.; Fernandes, T.J.; Guelfi, D. Technologies for Fertilizers and Management Strategies of N-Fertilization in Coffee Cropping Systems to Reduce Ammonia Losses by Volatilization. Plants 2022, 11, 3323. [Google Scholar] [CrossRef] [PubMed]
- Chagas, W.F.T.; Guelfi, D.R.; Caputo, A.L.C.; de Souza, T.L.; Andrade, A.B.; Faquin, V. Ammonia volatilization from blends with stabilized and controlled-released urea in the coffee system. Ciênc. Agrotecnologia 2016, 40, 497–509. [Google Scholar] [CrossRef]
- de Sousa Lima, J.; Andrade, O.V.S.; dos Santos, L.C.; de Morais, E.G.; Martins, G.S.; Mutz, Y.S.; Nascimento, V.L.; Marchiori, P.E.R.; Lopes, G.; Guilherme, L.R.G. Soybean Plants Exposed to Low Concentrations of Potassium Iodide Have Better Tolerance to Water Deficit through the Antioxidant Enzymatic System and Photosynthesis Modulation. Plants 2023, 12, 2555. [Google Scholar] [CrossRef]
- de Sousa Lima, J.; Andrade, O.V.S.; de Morais, E.G.; Machado, G.G.L.; dos Santos, L.C.; de Andrade, E.S.; Benevenute, P.A.N.; Martins, G.S.; Nascimento, V.L.; Marchiori, P.E.R.; et al. KI Increases Tomato Fruit Quality and Water Deficit Tolerance by Improving Antioxidant Enzyme Activity and Amino Acid Accumulation: A Priming Effect or Relief during Stress? Plants 2023, 12, 4023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, H.; Wang, M.; Zou, Z.; Zhou, P.; Wang, X.; Jin, J. A review of iodine in plants with biofortification: Uptake, accumulation, transportation, function, and toxicity. Sci. Total Environ. 2023, 878, 163203. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Photorespiration Process and Nitrogen Metabolism in Lettuce Plants (Lactuca sativa L.): Induced Changes in Response to Iodine Biofortification. J. Plant Growth Regul. 2010, 29, 477–486. [Google Scholar] [CrossRef]
- Smolen, S.; Sady, W. Influence of iodine fertilization and soil application of sucrose on the effectiveness of iodine biofortification, yield, nitrogen metabolism and biological quality of of spinach. Acta Sci. Pol. Hortorum Cultus 2011, 10, 51–63. [Google Scholar]
- Smoleń, S.; Sady, W.; Rożek, S.; Strzetelski, P.; Ledwożyw-Smoleń, I. Preliminary evaluation of the influence of iodine and nitrogen fertilization on the effectiveness of iodine biofortification and mineral composition of carrot storage roots. J. Elemntology 2011, 16, 275–285. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Kościk, B.; Skowrońska, M.; Kuśmierz, S.; Walczak, J.; Prażak, R. Quality of Rye Plants (Secale cereale) as Affected by Agronomic Biofortification with Iodine. Plants 2022, 12, 100. [Google Scholar] [CrossRef]
- Ramezani, S.; Yousefshahi, B.; Ramezan, D.; Zargar, M.; Pakina, E.; Bayat, M. Selenium, Iodine, and Supplementary Blue Light Enriched Fenugreek (Trigonella foenum-gracum L.) in Terms of Biochemical Quality, Mineral Uptake, and Trace Elements Accumulation in a Hydroponic System. Agriculture 2023, 13, 2009. [Google Scholar] [CrossRef]
- Mageshen, V.; Santhy, P. Effect of chitosan iodate complex biofortification on nutrient uptake in ‘shivam’ hybrid of tomato (Solanum lycopersicum L.). J. Appl. Nat. Sci. 2023, 15, 549–554. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Dos Anjos, L.H.C.; De Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; De Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brasil, 2018. [Google Scholar]
- IIUSS Working Group WRB (Ed.) World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization (FAO): Rome, Italy, 2015. [Google Scholar]
- Soil Survey Staff (Ed.) Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Lopes, A.S.; Guimarães Guilherme, L.R. A Career Perspective on Soil Management in the Cerrado Region of Brazil. Adv. Agron. 2016, 137, 1–72. [Google Scholar] [CrossRef]
- Agegnehu, G.; Amede, T.; Erkossa, T.; Yirga, C.; Henry, C.; Tyler, R.; Nosworthy, M.G.; Beyene, S.; Sileshi, G.W. Extent and management of acid soils for sustainable crop production system in the tropical agroecosystems: A review. Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 852–869. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brasil, 2017; ISBN 978-85-7035-771-7. [Google Scholar]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Sukor, A.S.A.; Adibah, A.M. Urea application in soil: Processes, losses, and alternatives—A review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Kalra, Y. Handbook of Reference Methods for Plant Analysis; Kalra, Y., Ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Arnhold, E. Easyanova: Analysis of Variance and Other Important Complementary Analyses 2019. Available online: https://cran.r-project.org/web/packages/easynova/ (accessed on 2 July 2024).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2022. Available online: https://www.r-project.org/ (accessed on 2 July 2024).
- Wei, T.; Simko, V.R. Package “corrplot”: Visualization of a Correlation Matrix 2017. Available online: https://cran.r-project.org/web/packages/corrplot/ (accessed on 2 July 2024).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Singh, B.P.; Wang, H.; Cai, X.; Chen, J.; Qin, H.; Li, Y.; Chang, S.X. Contrasting short-term responses of soil heterotrophic and autotrophic respiration to biochar-based and chemical fertilizers in a subtropical Moso bamboo plantation. Appl. Soil Ecol. 2021, 157, 103758. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, S.; Yin, M.; He, Z.; Bi, D. Co-pyrolysis of cellulose with urea and chitosan to produce nitrogen-containing compounds and nitrogen-doped biochar: Product distribution characteristics and reaction path analysis. J. Anal. Appl. Pyrolysis 2023, 169, 105795. [Google Scholar] [CrossRef]
- Raclavská, H.; Růžičková, J.; Raclavský, K.; Juchelková, D.; Kucbel, M.; Švédová, B.; Slamová, K.; Kacprzak, M. Effect of biochar addition on the improvement of the quality parameters of compost used for land reclamation. Environ. Sci. Pollut. Res. 2021, 30, 8563–8581. [Google Scholar] [CrossRef]
- Akaike, H. A Bayesian Extension of the Minimum AIC Procedure of Autoregressive Model Fitting. Biometrika 1979, 66, 237. [Google Scholar] [CrossRef]
- Suenaga, S.; Takano, Y.; Saito, T. Unraveling Binding Mechanism and Stability of Urease Inhibitors: A QM/MM MD Study. Molecules 2023, 28, 2697. [Google Scholar] [CrossRef] [PubMed]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a Nutritional Role of Iodine in Plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef] [PubMed]
- Adrade, O.V.S.; de Sousa Lima, J.; das Neves, T.T.; de Morais, E.G.; Benevenute, P.A.N.; dos Santos, L.C.; Nascimento, V.L.; Guilherme, L.R.G.; Marchiori, P.E.R. The Role of Potassium Iodate inMitigating the Damages of Water Deficit in Coffee Plants. J. Soil Sci. Plant Nutr. 2024, 1–7. [Google Scholar] [CrossRef]
| Parameters | Values | Protocol |
|---|---|---|
| pH (1 g of soil: 2.5 mL of water) | 4.5 | pH determined in water |
| Soil organic matter (g kg−1) | 24.9 | Walkley–Black method |
| Total nitrogen (g kg−1) | 2.3 | Kjeldahl method |
| Clay (g kg−1) | 670 | Boyoucos method |
| Silt (g kg−1) | 130 | Boyoucos method |
| Sand (g kg−1) | 200 | Boyoucos method |
| Exchangeable calcium 2+ (cmolc kg−1) | 0.4 | 1 mol L−1 KCl solution–soil test |
| Exchangeable magnesium2+ (cmolc kg−1) | 0.2 | 1 mol L−1 KCl solution–soil test |
| Available phosphorus (mg kg−1) | 0.4 | Mehlich-1 soil test |
| Available potassium (mg kg−1) | 24.8 | Mehlich-1 soil test |
| Available zinc (mg kg−1) | 0.2 | Mehlich-1 soil test |
| Available iron (mg kg−1) | 38.0 | Mehlich-1 soil test |
| Available manganese (mg kg−1) | 3.4 | Mehlich-1 soil test |
| Available copper (mg kg−1) | 1.2 | Mehlich-1 soil test |
| Available boron (mg kg−1) | 0.01 | Hot-water extraction method |
| Available sulfur (mg kg−1) | 2.9 | Monocalcium phosphate diluted in acetic acid method |
| Models | Iodine-Enriched Urea | Conventional Urea | Without Urea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | AIC | R2 | RMSE | AIC | R2 | RMSE | AIC | |
| Linear | 0.48 | 5.07 | 249.32 | 0.55 | 5.16 | 250.76 | 0.19 | 0.23 | 1.75 |
| Elovich | 0.75 | 3.48 | 219.18 | 0.84 | 3.11 | 210.25 | 0.16 | 0.23 | 3.00 |
| Exponential | 0.80 | 3.22 | 213.14 | 0.87 | 2.80 | 201.96 | 0.19 | 0.23 | 1.91 |
| Power function | 0.66 | 4.11 | 232.60 | 0.74 | 3.96 | 229.56 | 0.18 | 19.65 | 229.56 |
| Hyperbolic | 0.78 | 3.35 | 216.31 | 0.86 | 2.99 | 207.17 | 0.19 | 0.23 | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cezar, J.V.d.C.; Morais, E.G.d.; Lima, J.d.S.; Benevenute, P.A.N.; Guilherme, L.R.G. Iodine-Enriched Urea Reduces Volatilization and Improves Nitrogen Uptake in Maize Plants. Nitrogen 2024, 5, 891-902. https://doi.org/10.3390/nitrogen5040057
Cezar JVdC, Morais EGd, Lima JdS, Benevenute PAN, Guilherme LRG. Iodine-Enriched Urea Reduces Volatilization and Improves Nitrogen Uptake in Maize Plants. Nitrogen. 2024; 5(4):891-902. https://doi.org/10.3390/nitrogen5040057
Chicago/Turabian StyleCezar, João Victor da Costa, Everton Geraldo de Morais, Jucelino de Sousa Lima, Pedro Antônio Namorato Benevenute, and Luiz Roberto Guimarães Guilherme. 2024. "Iodine-Enriched Urea Reduces Volatilization and Improves Nitrogen Uptake in Maize Plants" Nitrogen 5, no. 4: 891-902. https://doi.org/10.3390/nitrogen5040057







