Contrasting Life-Form Influences Guam Ficus Foliar Nutrient Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Methods
2.2. Sample Handling and Analyses
3. Results
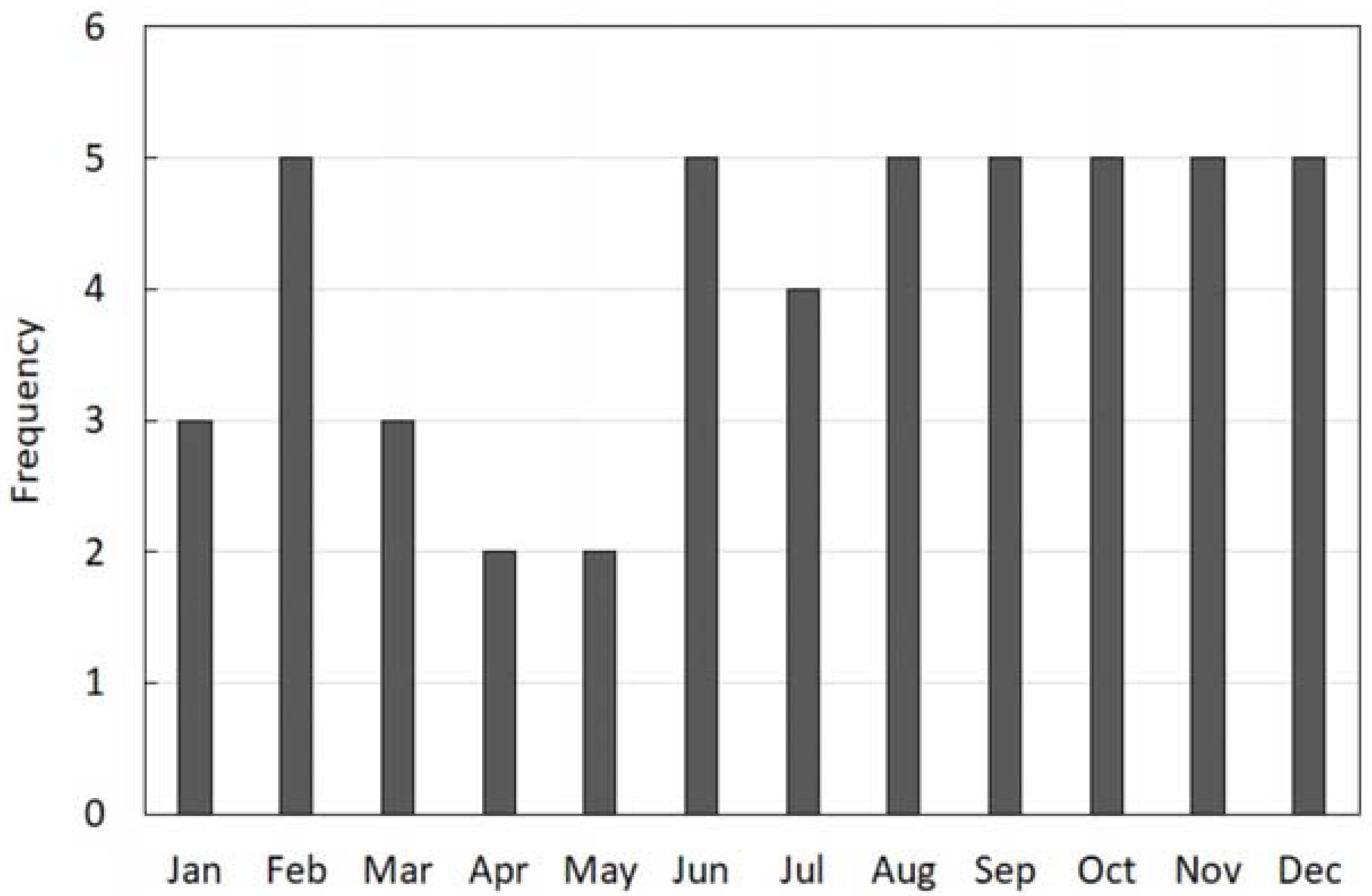
3.1. Phenology
3.2. Nutrient Concentration
3.3. Nutrient Stoichiometry
3.4. Nutrient Resorption Efficiency
4. Discussion
4.1. Nutrient Resorption
4.2. Nutrient Concentration and Stoichiometry
4.3. The Greatest Threats to Guam’s Ficus Tree Population
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonelli, A.; Fry, C.; Smith, R.J.; Eden, J.; Govaerts, R.H.A.; Kersey, P.; Lughadha, E.M.N.; Onstein, R.E.; Simmonds, M.S.J.; Zizka, A.; et al. State of the World’s Plants and Fungi 2023; Royal Botanic Gardens, Kew: Richmond, UK, 2023. [Google Scholar] [CrossRef]
- Rivers, M.; Newton, A.C.; Oldfield, S.; Global Tree Assessment Contributors. Scientists’ warning to humanity on tree extinctions. Plants People Planet 2023, 5, 466–482. [Google Scholar] [CrossRef]
- Botanic Gardens Conservation International. State of the World’s Trees; Botanic Gardens Conservation International: Richmond, UK, 2021. [Google Scholar]
- Raulerson, L.; Rinehart, A. Trees and Shrubs of the Northern Mariana Islands; Office of the Governor Commonwealth of the Northern Mariana Islands: Saipan, Northern Mariana Islands, 1991. [Google Scholar]
- Wiles, G.J.; Fujita, M.S. Food plants and economic importance of flying foxes on Pacific islands. Biol. Rep. 1992, 90, 24–35. [Google Scholar]
- Schreiner, I.H.; Nafus, D.M. Butterflies of Micronesia; University of Guam: Guam, USA, 1997. [Google Scholar]
- Gawel, A.M.; Rogers, H.S.; Miller, R.H.; Kerr, A.M. Contrasting ecological roles of non-native ungulates in a novel ecosystem. R. Soc. Open Sci. 2018, 5, 170151. [Google Scholar] [CrossRef]
- Donnegan, J.A.; Butler, S.L.; Grabowiecki, W.; Hiserote, B.A.; Limtiaco, D. Guam’s Forest Resources 2002; Resource Bulletin PNW-RB-243; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2004. [Google Scholar]
- Lazaro, M.; Kuegler, O.; Stanton, S.; Lehman, A.; Mafnas, J.; Yatskov, M. Guam’s Forest Resources: Forest Inventory and Analysis 2013; Resource Bulletin PNW-RB-270; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2020. [Google Scholar]
- Dobson, A.; Hutchinson, M.C.; Batterman, S. Plant communities and food webs. Front. Ecol. Evol. 2023, 11, 1253084. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: Key to search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Lü, X.-T.; Hu, Y.-Y.; Wolf, A.A.; Han, X.-G. Species richness mediates within-species nutrient resorption: Implications for the biodiversity–productivity relationship. J. Ecol. 2019, 107, 2346–2352. [Google Scholar] [CrossRef]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecolog. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 2021, 12, 4562. [Google Scholar] [CrossRef]
- Young, F.J. Soil Survey of Territory of Guam; U.S. Department of Agriculture Soil Conservation Service: Washington, DC, USA, 1988. [Google Scholar]
- National Oceanic and Atmospheric Administration. Local Climatological Data, Guam. Available online: https://www.weather.gov/gum (accessed on 2 October 2024).
- Marler, T.E. Pacific island tropical cyclones are more frequent and globally relevant, yet less studied. Front. Environ. Sci. 2014, 2, 42. [Google Scholar] [CrossRef]
- Stone, B.C. America’s Asiatic Flora: The plants of Guam. Am. Sci. 1971, 59, 308–319. [Google Scholar]
- Xu, M.; Zhu, Y.; Zhang, S.; Feng, Y.; Zhang, W.; Han, X. Global scaling the leaf nitrogen and phosphorus resorption of woody species: Revisiting some commonly held views. Sci. Total Environ. 2021, 788, 147807. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Fu, W.; Yuan, Z.; Yu, Q.; Peng, C.; Koerner, S.E.; Guo, L. Growing season temperature and precipitation affect nutrient resorption in herbaceous species through a foliar stoichiometric control strategy. Plant Soil 2023, 493, 45–60. [Google Scholar] [CrossRef]
- Sun, X.; Li, D.; Lü, X.; Fang, Y.; Ma, Z.; Wang, Z.; Chu, C.; Li, M.; Chen, H. Widespread controls of leaf nutrient resorption by nutrient limitation and stoichiometry. Funct. Ecol. 2023, 37, 1653–1662. [Google Scholar] [CrossRef]
- Lü, X.T.; Hou, S.L.; Reed, S.; Yin, J.X.; Hu, Y.Y.; Wei, H.W.; Zhang, Z.W.; Yang, G.J.; Liu, Z.Y.; Han, X.G. Nitrogen enrichment reduces nitrogen and phosphorus resorption through changes to species resorption and plant community composition. Ecosystems 2021, 24, 602–612. [Google Scholar] [CrossRef]
- Wang, P.; Fu, C.; Wang, L.; Yan, T. Delayed autumnal leaf senescence following nutrient fertilization results in altered nitrogen resorption. Tree Physiol. 2022, 42, 1549–1559. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar]
- Athokpam, F.D.; Garkoti, S.C. Dynamics of foliar nitrogen of evergreen and deciduous plant species in a wet tropical forest, South Assam, India. Plant Ecol. 2015, 216, 1117–1135. [Google Scholar] [CrossRef]
- Leopold, A.C. Senescence in plant development. Science 1961, 134, 1727–1732. [Google Scholar] [CrossRef]
- Ares, A.; Gleason, S.M. Foliar nutrients resorption in tree species. In New Research on Forest Ecology; Scaggs, A.K., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2007; pp. 1–32. [Google Scholar]
- Marler, T.E. Leaf elemental concentrations, stoichiometry, and resorption in Guam’s coastal karst forests. Diversity 2021, 13, 545. [Google Scholar] [CrossRef]
- Sardans, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. Recent advances and future research in ecological stoichiometry. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125611. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, M. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; de Ruiter, P.C. Species richness-productivity patterns differ between N-, P- and K-limited wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Wang, M.; Moore, T.R. Carbon, nitrogen, phosphorus, and potassium stoichiometry in an ombrotrophic peatland reflects plant functional type. Ecosystems 2014, 17, 673–684. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Xing, K.; Niinemets, Ü.; Rengel, Z.; Onoda, Y.; Xia, J.; Chen, H.Y.H.; Zhao, M.; Han, W.; Li, H. Global patterns of leaf construction traits and their covariation along climate and soil environmental gradients. New Phytol. 2021, 232, 1648–1660. [Google Scholar] [CrossRef]
- Ossola, A. Guam’s Ecological Fate Is in the Hands of the U.S. Military; National Geographic: Washington, DC, USA, 2018; Available online: https://www.nationalgeographic.com (accessed on 2 October 2024).
- Spies, N.P.; Mizerek, T.; Reeves, M.K.; Amidon, F.; Miller, S.E. Developed Systems in the Mariana Islands Archipelago. In Encyclopedia of the World’s Biomes; Goldstein, M.I., DellaSala, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 228–242. [Google Scholar] [CrossRef]
- Center for Biological Diversity and Prutehi Litekyan versus United States Department of Defense; del Toro, C.; United States Fish and Wildlife Service; Haaland, D. CIV 23-00019. Complaint for Declaratory and Injunctive Relief under the Endangered Species Act, Administrative Procedure Act, and Freedom of Information Act. United States District Court of Guam. 2023. Available online: https://www.biologicaldiversity.org/programs/biodiversity/pdfs/Camp-Blaz-Complaint.pdf (accessed on 2 October 2024).
- Owen, A. Guam culture, immigration and the US military build-up. Asia Pac. Viewp. 2010, 51, 304–318. [Google Scholar] [CrossRef]
- Marler, T.E.; Moore, A. Military threats to terrestrial resources not restricted to wartime: A case study from Guam. J. Environ. Sci. Eng. 2011, 5, 1198–1214. [Google Scholar]
- Copete, J.C.; Kik, A.; Novotny, V.; Cámara-Leret, R. The importance of Indigenous and local people for cataloging biodiversity. Trends Ecol. Evol. 2023, 38, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Kingston, L. The destruction of identity: Cultural genocide and indigenous peoples. J. Human Rights 2015, 14, 63–83. [Google Scholar] [CrossRef]
- Marler, T.E.; Wiecko, G.; Moore, A. Application of game theory to the interface between militarization and environmental stewardship in the Mariana Islands. Communic. Integr. Biol. 2012, 5, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. The intersection of a military culture and indigenous peoples in conservation issues. Communic. Integr. Biol. 2014, 7, e26665. [Google Scholar] [CrossRef]
- United Nations Commission on Human Rights. AL USA 7/2021. Available online: https://spcommreports.ohchr.org/TMResultsBase/DownLoadPublicCommunicationFile?gId=25885 (accessed on 2 October 2024).
- Schulze, P.C. Obstacles to Environmental Progress: A U.S. Perspective; UCL Press: London, UK, 2022. [Google Scholar]
- de Lima, R.A.F.; Phillips, O.L.; Duque, A.; Tello, S.; Davies, S.J.; de Oliveira, A.A.; Muller, S.; Coronado, E.N.H.; Vilanova, E.; Cuni-Sanchez, A.; et al. Making forest data fair and open. Nat. Ecol. Evol. 2022, 6, 656–658. [Google Scholar] [CrossRef]
- Mabry, M.E.; Caomhanach, N.; Abrahams, R.S.; Gaynor, M.L.; Pham, K.K.; Williams, T.M.; Murphy, K.S.; Smocovitis, V.B.; Soltis, D.E.; Soltis, P.S. Building an inclusive botany: The “radicle” dream. Plants People Planet 2024, 6, 544–557. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Kreft, H.; Irl, S.D.H.; Norder, S.; Ah-Peng, C.; Borges, P.A.V.; Burns, K.C.; de Nascimento, L.; Meyer, J.-Y.; Montes, E.; et al. Scientists’ warning—The outstanding biodiversity of islands is in peril. Global Ecol. Conserv. 2021, 31, e01847. [Google Scholar] [CrossRef]
- Nowak, K.; Bear, D.; Dutta, A.; Traphagen, M.; Zmihorski, M.; Jaroszewicz, B. Threats to conservation from national security interests. Conserv. Biol. 2024, 38, e14193. [Google Scholar] [CrossRef]



| Variable | Species f 1,48 | Species p | Stage f 3,48 | Stage p | Interaction f 3,48 | Interaction p |
|---|---|---|---|---|---|---|
| Nitrogen | 377.866 | <0.001 | 289.405 | <0.001 | 79.079 | <0.001 |
| Phosphorus | 217.307 | <0.001 | 169.312 | <0.001 | 67.781 | <0.001 |
| Potassium | 280.305 | <0.001 | 298.062 | <0.001 | 57.804 | <0.001 |
| Variable | Species f 1,48 | Species p | Stage f 3,48 | Stage p | Interaction f 3,48 | Interaction p |
|---|---|---|---|---|---|---|
| N:K | 6.732 | 0.013 | 23.421 | <0.001 | 1.974 | 0.130 |
| N:P | 14.507 | <0.001 | 18.368 | <0.001 | 4.364 | 0.009 |
| K:P | 1.591 | 0.213 | 7.553 | <0.001 | 12.253 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E. Contrasting Life-Form Influences Guam Ficus Foliar Nutrient Dynamics. Nitrogen 2024, 5, 915-926. https://doi.org/10.3390/nitrogen5040059
Marler TE. Contrasting Life-Form Influences Guam Ficus Foliar Nutrient Dynamics. Nitrogen. 2024; 5(4):915-926. https://doi.org/10.3390/nitrogen5040059
Chicago/Turabian StyleMarler, Thomas E. 2024. "Contrasting Life-Form Influences Guam Ficus Foliar Nutrient Dynamics" Nitrogen 5, no. 4: 915-926. https://doi.org/10.3390/nitrogen5040059
APA StyleMarler, T. E. (2024). Contrasting Life-Form Influences Guam Ficus Foliar Nutrient Dynamics. Nitrogen, 5(4), 915-926. https://doi.org/10.3390/nitrogen5040059





