Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security
Abstract
:1. Introduction
| N-Fixing Symbioses | Nitrogen Fixing Rate (kg N·ha1·year−1) | Nitrogen-Fixer and Total Number | Main Distribution Area | References |
|---|---|---|---|---|
| Mesorhizobium ephedrae | 850 | Ephedra (5 species) | Tropical low pH environments in Malaysia and the Western Pacific | [26,27] |
| Legumes/rhizobia | 300~400 | Brassinae (550~660) | Humid tropics | [28,29] |
| Mimosineae (88~102) | Tropical subtropics (Asia, Africa, Australia, and North America) | |||
| Papilioninae (921~973) | Woody individuals are mainly found in tropical subtropics, while herbs are mostly found in temperate and boreal forests | |||
| Actinomycorrhizal plants | 15~90 | Betulaceae, Casuarinaceae and Myriceaceae Rosaceae, rhamnaceae and Elaeagniaceae Masanaceae and four trees (200) | Temperate and high northern latitudes | [30,31] |
| Plants/cyanobacteria | 2~41 | Lichen (moss), fern | Extreme environments (deserts, grasslands, and frozen soils) | [32,33] |
| — | Cycads alone (more than 250) | Arid woodland of Australia and South Africa | [34] | |
| 72 | Rhizome or rhizomes, petiole only (about 50) | Found naturally in the tropical humid mountains of the Southern Hemisphere (Hawaii to the central and southern United States, New Zealand, Southeast Asia, and the southernmost parts of South America) | [33] |
2. Nitrogen Fixation Mechanisms
2.1. Advances in Agriculture with Cyanobacteria Nitrogen Fixation Techniques
2.2. Molecular Mechanisms of Nitrogen Fixation in Cyanobacteria
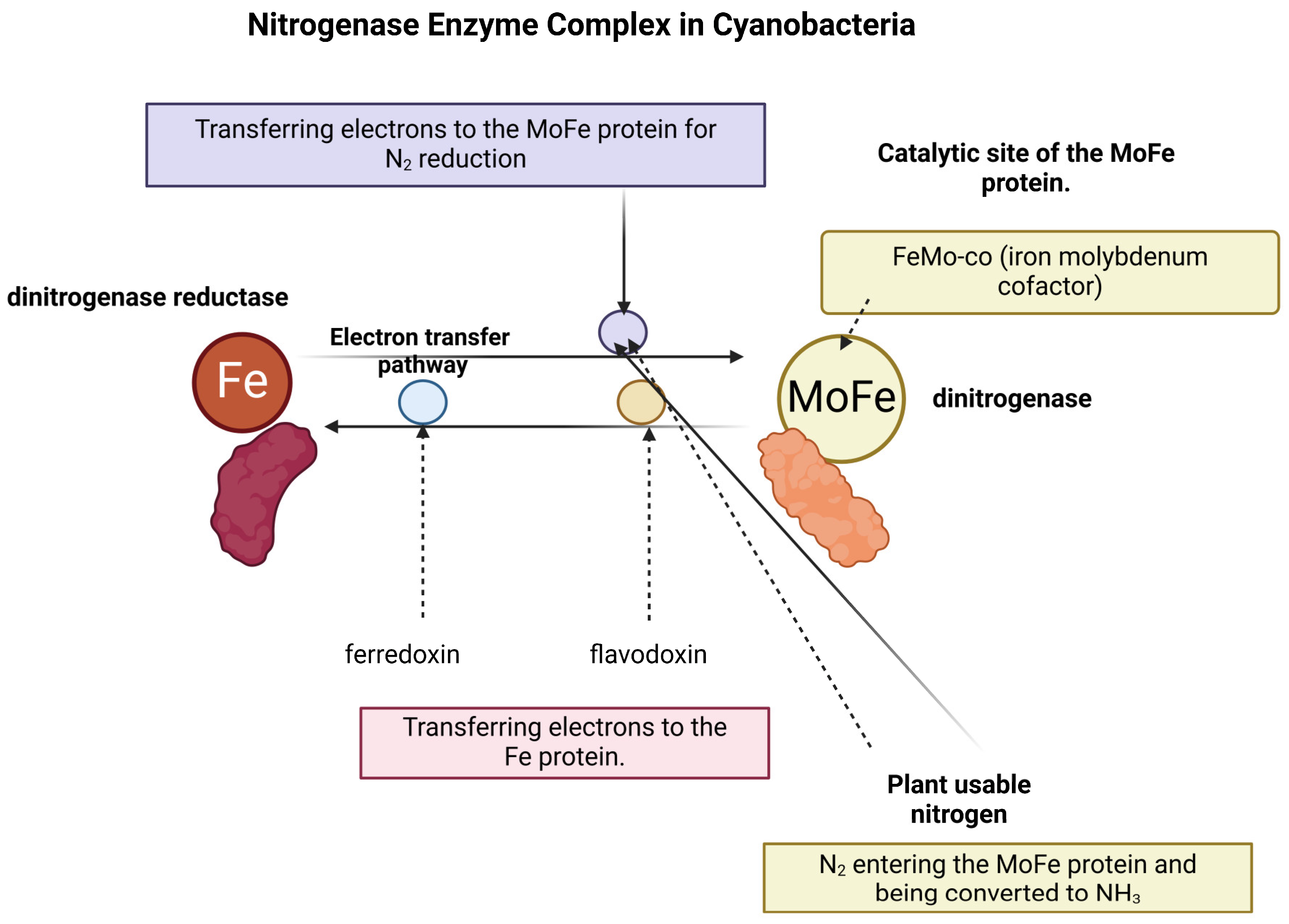
2.3. Nitrogenase Enzyme Complex
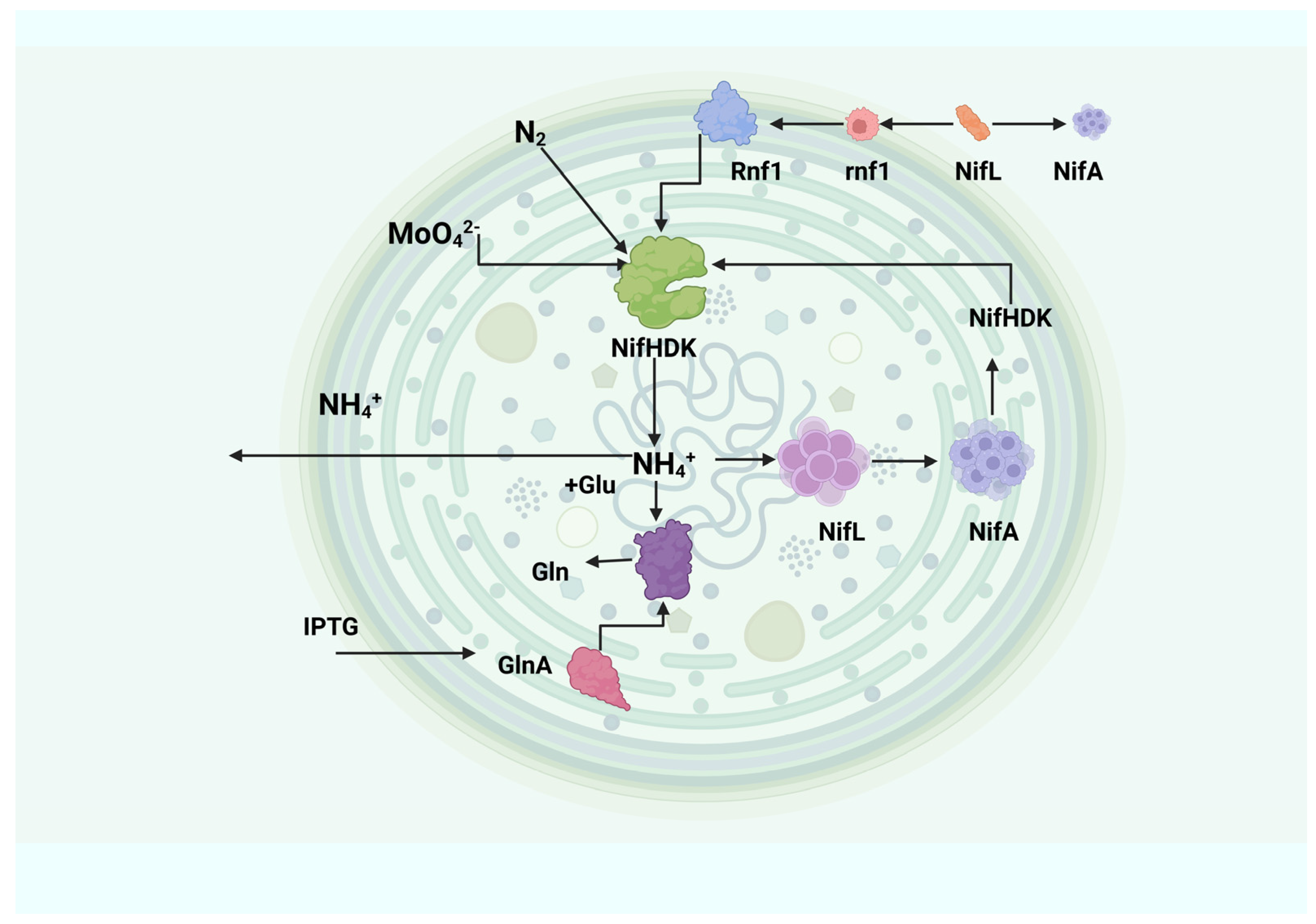
2.4. Nitrogenase Genes and Regulation
2.5. Adaptations for Nitrogen Fixation in Cyanobacteria
3. Genetic and Biochemical Factors
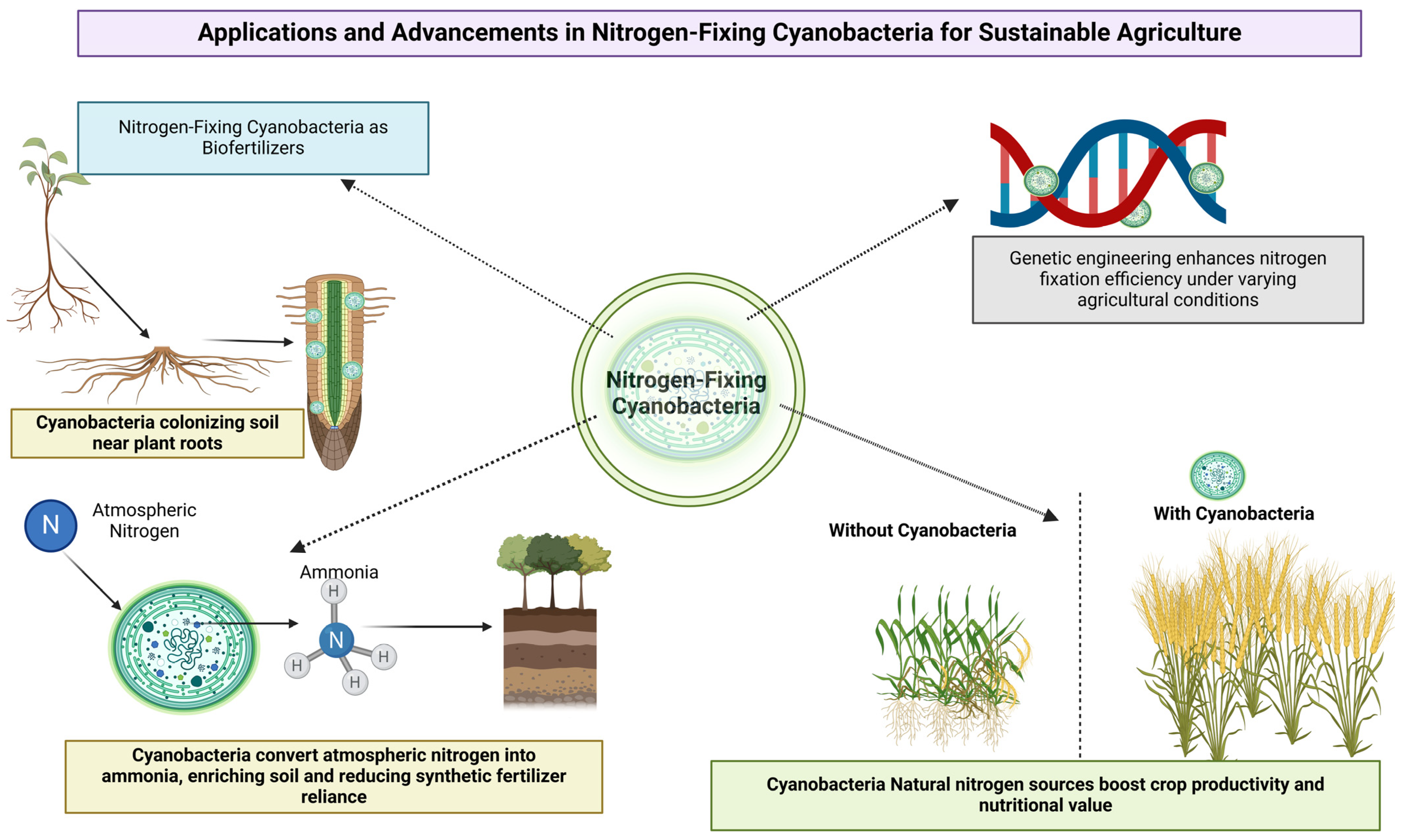
4. Biotechnological Applications
4.1. Nitrogen-Fixing Cyanobacteria as Biofertilizers
4.2. Improving Crop Yield and Quality
4.3. Genetic Engineering Approaches for Enhanced Nitrogen Fixation
4.4. Strain Improvement Strategies
4.5. The Role of Mixed Inoculation with Other Beneficial Organisms
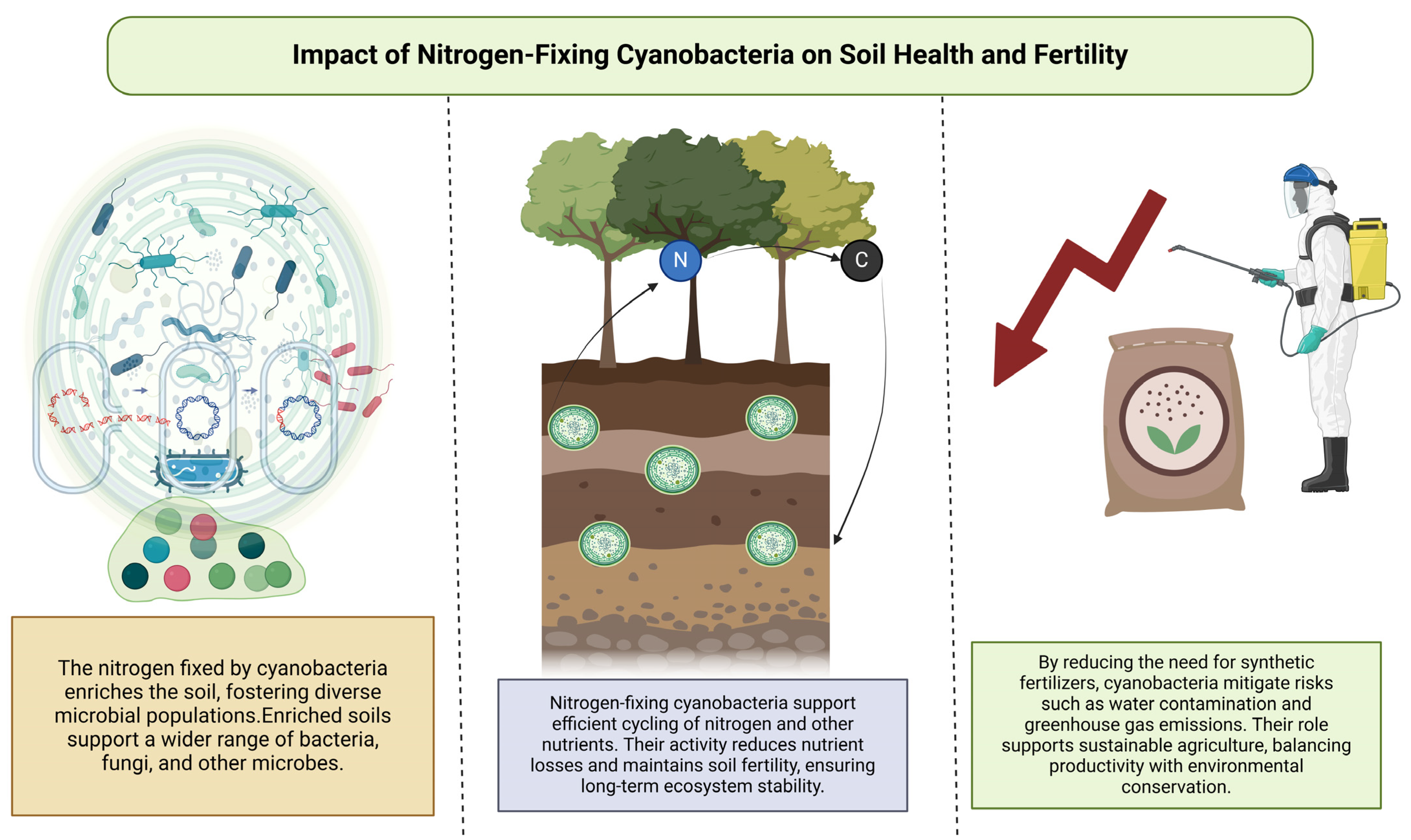
5. Impact on Soil Health
5.1. Influence of Nitrogen-Fixing Cyanobacteria on Soil Microbial Communities
5.2. Soil Fertility Enhancement and Nutrient Cycling
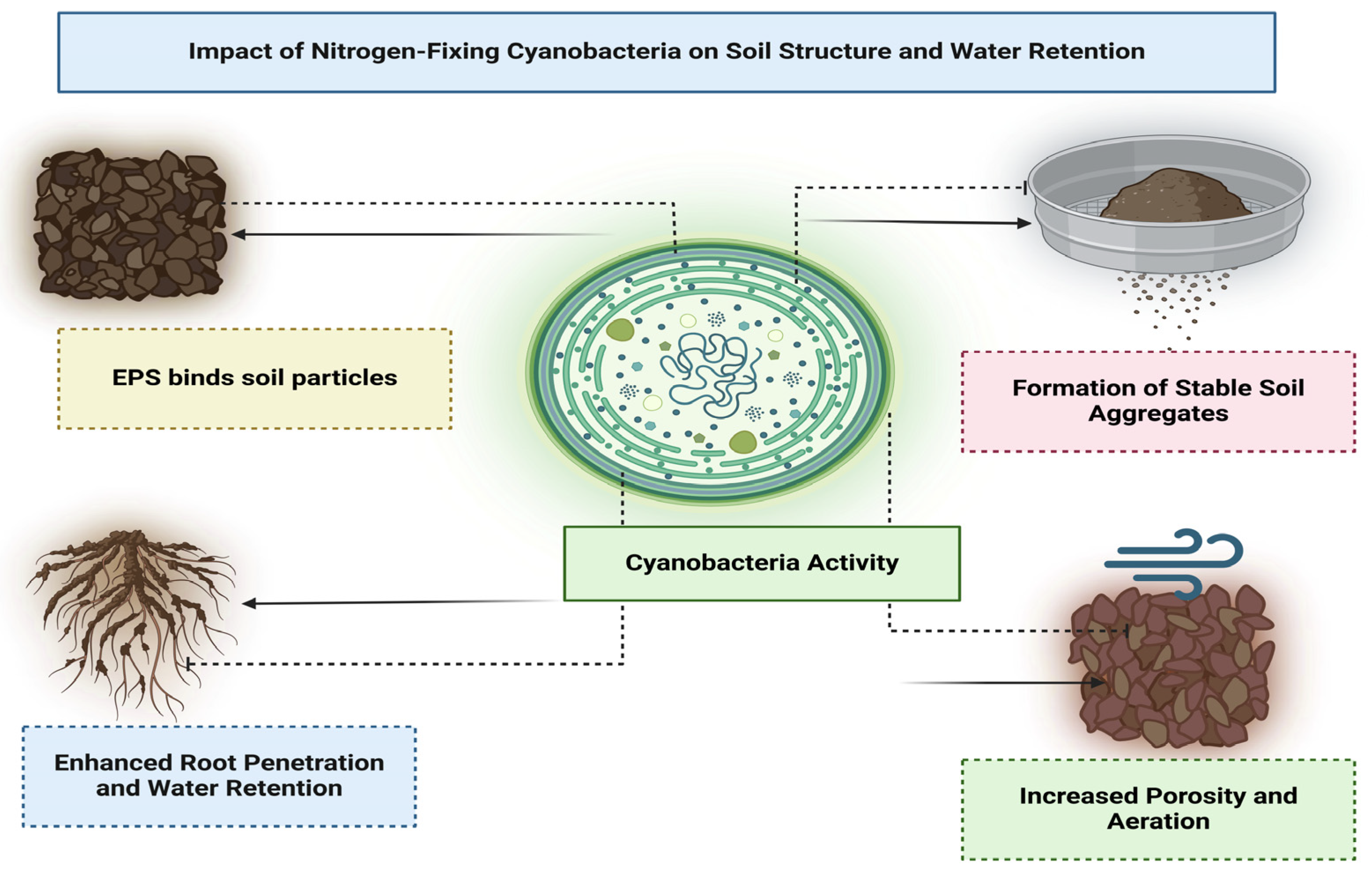
5.3. Effects on Soil Structure and Water Retention
6. Environmental Sustainability
6.1. Reduction of Synthetic Nitrogen Fertilizer Usage
6.2. Mitigation of Nitrogen Runoff and Environmental Pollution
6.3. Carbon Sequestration and Soil Health
7. Challenges and Limitations
7.1. Oxygen Sensitivity of Nitrogenase and Strategies to Overcome It
7.2. Competition with Native Microorganisms
7.3. Field Implementation Challenges and Risks
8. Future Directions
8.1. Advances in Synthetic Biology for Cyanobacterial Nitrogen Fixation
8.2. Integration with Other Agricultural Practices for Maximum Impact
8.3. Scaling-Up Strategies for Large-Scale Agriculture
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mrabet, R. Sustainable agriculture for food and nutritional security. In Sustainable Agriculture and the Environment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–90. [Google Scholar]
- Singh, B.; Ryan, J. Managing Fertilizers to Enhance Soil Health; International Fertilizer Industry Association: Paris, France, 2015; Volume 1. [Google Scholar]
- Verdenal, T.; Dienes-Nagy, Á.; Spangenberg, J.E.; Zufferey, V.; Spring, J.-L.; Viret, O.; Marin-Carbonne, J.; Van Leeuwen, C. Understanding and managing nitrogen nutrition in grapevine: A review. Oeno One 2021, 55, 1–43. [Google Scholar] [CrossRef]
- Soetan, K.; Olaiya, C.; Oyewole, O. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Romman, M.; Parvez, R.; Adnan, M.; Gul, F.; Haroon, M.; Ullah, R.; Saud, S.; Ahmed, N.; Hameed, I.; Nawaz, T. Nitrogen-fixing Biofertilizers. In Biofertilizers for Sustainable Soil Management; CRC Press: Boca Raton, FL, USA, 2023; pp. 233–254. [Google Scholar]
- Smil, V. Feeding the World: A Challenge for the Twenty-First Century; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Nawaz, T.; Junaid, M.; Kanwal, M.; Ahmed, S.; Ahmed, N.; Ullah, R.; Adnan, M.; Saeed, M.; Romman, M.; Fahad, S. 15 Bio-fertilizer Effects on Plant-parasitic. In Biofertilizers for Sustainable Soil Management; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Sahu, S.; Arya, S.K. Mitigation of greenhouse gas emissions in agriculture and food processing through sustainable management practices for climate change. In Advances and Technology Development in Greenhouse Gases: Emission, Capture and Conversion; Elsevier: Amsterdam, The Netherlands, 2024; pp. 71–96. [Google Scholar]
- Verma, R.C.; Singh, N.; Gangavati, A.R.; Ashoka, P.; Kesarwani, A.; Ali, I.; Pandey, S.K. A Review of Long-Term Effects of Mineral Fertilizers on Soil Microorganisms. Int. J. Plant Soil Sci. 2023, 35, 1145–1155. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Gutschick, V.P. Long-Term Strategies for Supplying Nitrogen to Crops; Los Alamos National Lab (LANL): Los Alamos, NM, USA, 1977. [Google Scholar]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Ullah, I.; Dawar, K.; Tariq, M.; Sharif, M.; Fahad, S.; Adnan, M.; Ilahi, H.; Nawaz, T.; Alam, M.; Ullah, A. Gibberellic acid and urease inhibitor optimize nitrogen uptake and yield of maize at varying nitrogen levels under changing climate. Environ. Sci. Pollut. Res. 2021, 29, 6568–6577. [Google Scholar] [CrossRef]
- de Vries, W. Impacts of nitrogen emissions on ecosystems and human health: A mini review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Khan, F.U.; Khan, A.A.; Li, K.; Xu, X.; Adnan, M.; Fahad, S.; Ahmad, R.; Khan, M.A.; Nawaz, T.; Zaman, F. Influences of long-term crop cultivation and fertilizer management on soil aggregates stability and fertility in the Loess Plateau, Northern China. J. Soil Sci. Plant Nutr. 2022, 22, 1446–1457. [Google Scholar] [CrossRef]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Rikkinen, J. Cyanobacteria in terrestrial symbiotic systems. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Springer: Cham, Switzerland, 2017; pp. 243–294. [Google Scholar]
- Adams, D.G.; Bergman, B.; Nierzwicki-Bauer, S.; Rai, A.; Schüßler, A. Cyanobacterial-plant symbioses. Prokaryotes A Handb. Biol. Bact. 2006, 1, 331–363. [Google Scholar]
- Prasanna, R.; Sood, A.; Ratha, S.K.; Singh, P.K. Cyanobacteria as a “green” option for sustainable agriculture. Cyanobacteria Econ. Perspect. 2014, 145–166. [Google Scholar]
- Devaprakash, M.; Thirumalaivasan, R.; Sivakumar, N.; Shyamkumar, R. Cyanobacterial interactions and symbiosis. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2024; pp. 425–489. [Google Scholar]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.; Poole, P.S. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [PubMed]
- Bohlool, B.; Ladha, J.; Garrity, D.; George, T. Biological nitrogen fixation for sustainable agriculture: A perspective. Plant Soil 1992, 141, 1–11. [Google Scholar]
- Pathak, J.; Rajneesh; Maurya, P.K.; Singh, S.P.; Haeder, D.-P.; Sinha, R.P. Cyanobacterial farming for environment friendly sustainable agriculture practices: Innovations and perspectives. Front. Environ. Sci. 2018, 6, 7. [Google Scholar]
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; O’Donnell, C.; Ktenioudaki, A. Global food security–Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20. [Google Scholar]
- Singh, P.K.; Fillat, M.F.; Kumar, A. Cyanobacterial Lifestyle and Its Applications in Biotechnology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Dupin, S.E.; Geurts, R.; Kiers, E.T. The non-legume Parasponia andersonii mediates the fitness of nitrogen-fixing rhizobial symbionts under high nitrogen conditions. Front. Plant Sci. 2020, 10, 1779. [Google Scholar]
- Gresshoff, P.M. Molecular Biology of Symbiotic Nitrogen Fixation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Pankievicz, V.C.; Irving, T.B.; Maia, L.G.; Ané, J.-M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 2019, 17, 99. [Google Scholar]
- Hu, B.; Flemetakis, E.; Liu, Z.; Hänsch, R.; Rennenberg, H. Significance of nitrogen-fixing actinorhizal symbioses for restoration of depleted, degraded, and contaminated soil. Trends Plant Sci. 2023, 28, 752–764. [Google Scholar]
- Moura, E.G.; Carvalho, C.S.; Bucher, C.P.; Souza, J.L.; Aguiar, A.C.; Ferraz Junior, A.S.; Bucher, C.A.; Coelho, K.P. Diversity of Rhizobia and importance of their interactions with legume trees for feasibility and sustainability of the tropical agrosystems. Diversity 2020, 12, 206. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Bergman, B. Why does Gunnera do it and other angiosperms don’t? An evolutionary perspective on the Gunnera–Nostoc Symbiosis. In Prokaryotic Symbionts in Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 207–224. [Google Scholar]
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B. Towards an ecological understanding of biological nitrogen fixation. Nitrogen Cycle Reg. Glob. Scales 2002, 57, 1–45. [Google Scholar]
- Barminski, R.; Storteboom, H.; Davis, J.G. Development and evaluation of an organically certifiable growth medium for cultivation of cyanobacteria. J. Appl. Phycol. 2016, 28, 2623–2630. [Google Scholar] [CrossRef]
- Silva, P.G.; Silva, H.D.J. Biomass production of Tolypothrix tenuis as a basic component of a cyanobacterial biofertilizer. J. Appl. Phycol. 2013, 25, 1729–1736. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Garrison, G.L.; Biermacher, J.T.; Brorsen, B.W. How much will large-scale production of cell-cultured meat cost? J. Agric. Food Res. 2022, 10, 100358. [Google Scholar] [CrossRef]
- Mathesius, U. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Akoijam, C.; Singh, A.K.; Rai, A.N. Characterization of free-living cyanobacterial strains and their competence to colonize rice roots. Biol. Fertil. Soils 2012, 48, 679–687. [Google Scholar] [CrossRef]
- Bond, W.; Grundy, A. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [Google Scholar] [CrossRef]
- Tang, Q.; Cotton, A.; Wei, Z.; Xia, Y.; Daniell, T.; Yan, X. How does partial substitution of chemical fertiliser with organic forms increase sustainability of agricultural production? Sci. Total Environ. 2022, 803, 149933. [Google Scholar] [PubMed]
- Xu, P.; Wang, E. Diversity and regulation of symbiotic nitrogen fixation in plants. Curr. Biol. 2023, 33, R543–R559. [Google Scholar] [PubMed]
- Fuchs, W.; Rachbauer, L.; Rittmann, S.K.-M.; Bochmann, G.; Ribitsch, D.; Steger, F. Eight Up-Coming Biotech Tools to Combat Climate Crisis. Microorganisms 2023, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Zumft, W.; Mortenson, L. The nitrogen-fixing complex of bacteria. Biochim. Biophys. Acta (BBA)-Rev. Bioenerg. 1975, 416, 1–52. [Google Scholar]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar]
- Einsle, O.; Rees, D.C. Structural enzymology of nitrogenase enzymes. Chem. Rev. 2020, 120, 4969–5004. [Google Scholar]
- Hentschel, U.; Piel, J.; Degnan, S.M.; Taylor, M.W. Genomic insights into the marine sponge microbiome. Nat. Rev. Microbiol. 2012, 10, 641–654. [Google Scholar]
- Berman-Frank, I.; Lundgren, P.; Falkowski, P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 2003, 154, 157–164. [Google Scholar]
- Nawaz, T.; Joshi, N.; Nelson, D.; Saud, S.; Abdelsalam, N.R.; Abdelhamid, M.M.; Jaremko, M.; Rahman, T.U.; Fahad, S. Harnessing the potential of nitrogen-fixing cyanobacteria: A rich bio-resource for sustainable soil fertility and enhanced crop productivity. Environ. Technol. Innov. 2024, 36, 103886. [Google Scholar]
- Yang, N.; Lin, Y.-A.; Merkel, C.A.; DeMers, M.A.; Qu, P.-P.; Webb, E.A.; Fu, F.-X.; Hutchins, D.A. Molecular mechanisms underlying iron and phosphorus co-limitation responses in the nitrogen-fixing cyanobacterium Crocosphaera. ISME J. 2022, 16, 2702–2711. [Google Scholar]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas reinhardtii, a Reference Organism to Study Algal–Microbial Interactions: Why Can’t They Be Friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.J. Multiscale, Multiparadigm Metabolic Modeling of the Keystone Diazotrophic Cyanobacterium, Trichodesmium erythraeum; Colorado School of Mines: Golden, CO, USA, 2019. [Google Scholar]
- Singh, R.S.; Singh, P.; Verma, R.L.; Jena, D.; Kumar, A.; Singh, O.N. Biotechnology and Genomics-Based Strategies for Enhancing Photosynthetic Capacity and Nutrient-Use Efficiency of Crops. In Handbook of Energy Management in Agriculture; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–23. [Google Scholar]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.E.; Kobayashi, H.; Walker, G.C. Molecular determinants of a symbiotic chronic infection. Annu. Rev. Genet. 2008, 42, 413–441. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.; Muro-Pastor, A.M.; Flores, E. Nitrogen control in cyanobacteria. J. Bacteriol. 2001, 183, 411–425. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar]
- Fay, P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 1992, 56, 340–373. [Google Scholar] [CrossRef]
- Paerl, H.W.; Pinckney, J.L.; Steppe, T.F. Cyanobacterial–bacterial mat consortia: Examining the functional unit of microbial survival and growth in extreme environments. Environ. Microbiol. 2000, 2, 11–26. [Google Scholar] [CrossRef]
- Haselkorn, R. Heterocyst Differentiation and Nitrogen Fixation in Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5, pp. 233–255. [Google Scholar]
- Serraj, R.; Vadez, V.; Sinclair, T. Feedback regulation of symbiotic N2 fixation under drought stress. Agronomie 2001, 21, 621–626. [Google Scholar] [CrossRef]
- Leigh, J.A.; Dodsworth, J.A. Nitrogen regulation in bacteria and archaea. Annu. Rev. Microbiol. 2007, 61, 349–377. [Google Scholar] [CrossRef]
- Dahal, P. Polysaccharide-Based Hydrogel Beads for Biological Nitrogen Fixation. Master’s Thesis, South Dakota State University, Brookings, SD, USA, 2023. [Google Scholar]
- Herrero, A.; Flores, E. The cyanobacteria: Molecular biology, genomics, and evolution. Phycologia 2008, 47, 236–237. [Google Scholar]
- Kaplan-Levy, R.N.; Hadas, O.; Summers, M.L.; Rücker, J.; Sukenik, A. Akinetes: Dormant cells of cyanobacteria. Dormancy Resist. Harsh Environ. 2010, 21, 5–27. [Google Scholar]
- Ho, H.-I.; Park, C.-H.; Yoo, K.-E.; Kim, N.-Y.; Hwang, S.-J. Survival and development strategies of cyanobacteria through akinete formation and germination in the life cycle. Water 2024, 16, 770. [Google Scholar] [CrossRef]
- Garg, R.; Maldener, I. The formation of spore-like akinetes: A survival strategy of filamentous cyanobacteria. Microb. Physiol. 2021, 31, 296–305. [Google Scholar]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2010, 2, a000315. [Google Scholar]
- Montesinos, M.a.L.; Muro-Pastor, A.M.a.; Herrero, A.; Flores, E. Ammonium/Methylammonium Permeases of a Cyanobacterium: Identification and Analysis of Three Nitrogen-Regulated amt Genes in Synechocystis sp. PCC 6803. J. Biol. Chem. 1998, 273, 31463–31470. [Google Scholar] [CrossRef]
- Klähn, S.; Hagemann, M. Compatible solute biosynthesis in cyanobacteria. Environ. Microbiol. 2011, 13, 551–562. [Google Scholar] [CrossRef]
- Schwarz, R.; Forchhammer, K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: Emergence of a complex network of cellular responses. Microbiology 2005, 151, 2503–2514. [Google Scholar]
- Peters, G.; Meeks, J. The Azolla-Anabaena symbiosis: Basic biology. Annu. Rev. Plant Biol. 1989, 40, 193–210. [Google Scholar] [CrossRef]
- Carrapiço, F. Azolla as a superorganism. Its implication in symbiotic studies. Symbioses Stress Jt. Ventur. Biol. 2010, 17, 225–241. [Google Scholar]
- Mandal, B.; Vlek, P.; Mandal, L. Beneficial effects of blue-green algae and Azolla, excluding supplying nitrogen, on wetland rice fields: A review. Biol. Fertil. Soils 1999, 28, 329–342. [Google Scholar] [CrossRef]
- Qadir, M.; Iqbal, A.; Hussain, A.; Hussain, A.; Shah, F.; Yun, B.-W.; Mun, B.-G. Exploring Plant–Bacterial Symbiosis for Eco-Friendly Agriculture and Enhanced Resilience. Int. J. Mol. Sci. 2024, 25, 12198. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.C.; Elhai, J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. Rev. 2002, 66, 94–121. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Söderbäck, E.; Bergman, B. Cyanobacterium-plant symbioses. New Phytol. 2000, 147, 449–481. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Matinha-Cardoso, J.; Tamagnini, P.; Oliveira, P. Extracellular vesicles: An overlooked secretion system in cyanobacteria. Life 2020, 10, 129. [Google Scholar] [CrossRef]
- Shantharam, S.; Mattoo, A.K. Enhancing biological nitrogen fixation: An appraisal of current and alternative technologies for N input into plants. Plant Soil 1997, 194, 205–216. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef]
- Singhal, R.K.; Fahad, S.; Kumar, P.; Choyal, P.; Javed, T.; Jinger, D.; Singh, P.; Saha, D.; Md, P.; Bose, B. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 2023, 100, 237–265. [Google Scholar] [CrossRef]
- Howard, J.B.; Rees, D.C. How many metals does it take to fix N2? A mechanistic overview of biological nitrogen fixation. Proc. Natl. Acad. Sci. USA 2006, 103, 17088–17093. [Google Scholar] [CrossRef]
- Burén, S.; Jiménez-Vicente, E.; Echavarri-Erasun, C.; Rubio, L.M. Biosynthesis of nitrogenase cofactors. Chem. Rev. 2020, 120, 4921–4968. [Google Scholar] [CrossRef]
- Zeng, Y.; Priest, C.; Wang, G.; Wu, G. Restoring the nitrogen cycle by electrochemical reduction of nitrate: Progress and prospects. Small Methods 2020, 4, 2000672. [Google Scholar] [CrossRef]
- Gu, L.; Nawaz, T.; Qiu, Y.; Wu, Y.; Zhou, R. Photosynthetic conversion of CO2 and H2O to long-chain terpene alcohol by genetically engineered N2-fixing cyanobacteria. In Photosynthesis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 451–461. [Google Scholar]
- Rees, D.C.; Chan, M.K.; Kim, J. Structure and function of nitrogenase. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 1993; Volume 40, pp. 89–119. [Google Scholar]
- Stiefel, E.I.; George, G.N. Ferredoxins, hydrogenases, and nitrogenases: Metal-sulfide proteins. In Bioinorganic Chemistry; Bertini, I., Gray, H.B., Lippard, S.J., Valentine, J.S., Eds.; University Science Books: Herndon, VA, USA, 1994; pp. 365–453. [Google Scholar]
- Chalkley, M.J.; Drover, M.W.; Peters, J.C. Catalytic N2-to-NH3 (or-N2H4) conversion by well-defined molecular coordination complexes. Chem. Rev. 2020, 120, 5582–5636. [Google Scholar] [CrossRef] [PubMed]
- Danyal, K. Electron Transfer and Substrate Reduction in Nitrogenase. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2014. [Google Scholar]
- Gaughan, S.J. Effect of Oriented Electric Fields on Biologically Relevant Iron–Sulfur Clusters and Bioinformatics Investigations of Biotin Synthase. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2023. [Google Scholar]
- Schimpl, J.; Petrilli, H.M.; Blöchl, P.E. Nitrogen binding to the FeMo-cofactor of nitrogenase. J. Am. Chem. Soc. 2003, 125, 15772–15778. [Google Scholar] [CrossRef] [PubMed]
- Osyczka, A.; Moser, C.C.; Daldal, F.; Dutton, P.L. Reversible redox energy coupling in electron transfer chains. Nature 2004, 427, 607–612. [Google Scholar] [CrossRef]
- Khanna, N.; Lindblad, P. Cyanobacterial hydrogenases and hydrogen metabolism revisited: Recent progress and future prospects. Int. J. Mol. Sci. 2015, 16, 10537–10561. [Google Scholar] [CrossRef]
- Debnath, S.; Rawat, D.; Mukherjee, A.K.; Adhikary, S.; Kundu, R. Applications and constraints of plant beneficial microorganisms in agriculture. In Biostimulants in Plant Science; IntechOpen: London, UK, 2019. [Google Scholar]
- Nawaz, T.; Hassan, S.; Ur Rahman, T.; Khan, M.N.R.; Fahad, S.; Saleem, A.; Khan, I.; Saud, S. Harnessing Cyanobacteria: Nitrogen fixation and its impact on climate and plant growth. In Environment, Climate, Plant and Vegetation Growth; Springer: Berlin/Heidelberg, Germany, 2024; pp. 41–73. [Google Scholar]
- Rosa, L.; Gabrielli, P. Energy and food security implications of transitioning synthetic nitrogen fertilizers to net-zero emissions. Environ. Res. Lett. 2022, 18, 014008. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Tully, K.; Ryals, R. Nutrient cycling in agroecosystems: Balancing food and environmental objectives. Agroecol. Sustain. Food Syst. 2017, 41, 761–798. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef]
- Mohanty, L.K.; Singh, N.; Raj, P.; Prakash, A.; Tiwari, A.K.; Singh, V.; Sachan, P. Nurturing crops, enhancing soil health, and sustaining agricultural prosperity worldwide through agronomy. J. Exp. Agric. Int. 2024, 46, 46–67. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsanaa, T.; Bharghavi, A.; Jayamuthunagai, J.; Praveenkumar, R. Biohydrogen and Biogas—An overview on feedstocks and enhancement process. Fuel 2016, 185, 810–828. [Google Scholar]
- Mahawar, H.; Prasanna, R. Cyanobacteria and their Associations as Promising Options for Climate-resilient Agriculture. J. Indian Soc. Soil Sci. 2024, 72, 1209–1224. [Google Scholar]
- Ng, Z.Y.; Ajeng, A.A.; Cheah, W.Y.; Ng, E.-P.; Abdullah, R.; Ling, T.C. Towards circular economy: Potential of microalgae–bacterial-based biofertilizer on plants. J. Environ. Manag. 2024, 349, 119445. [Google Scholar]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [PubMed]
- Meeks, J.C. Symbiosis between nitrogen-fixing cyanobacteria and plants. BioScience 1998, 48, 266–276. [Google Scholar]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar]
- Govindasamy, R.; Gayathiri, E.; Sankar, S.; Venkidasamy, B.; Prakash, P.; Rekha, K.; Savaner, V.; Pari, A.; Thirumalaivasan, N.; Thiruvengadam, M. Emerging trends of nanotechnology and genetic engineering in cyanobacteria to optimize production for future applications. Life 2022, 12, 2013. [Google Scholar] [CrossRef]
- Sessitsch, A.; Howieson, J.; Perret, X.; Antoun, H.; Martínez-Romero, E. Advances in Rhizobium research. Crit. Rev. Plant Sci. 2002, 21, 323–378. [Google Scholar]
- Ibáñez, A.; Garrido-Chamorro, S.; Vasco-Cárdenas, M.F.; Barreiro, C. From lab to field: Biofertilizers in the 21st century. Horticulturae 2023, 9, 1306. [Google Scholar] [CrossRef]
- Reddy, M.B.; Sravani, P.; Kumar, S.; Rajawat, M.V.S.; Jaiswal, D.K.; Dhar, S.; Azman, E.A.; Garg, K.; Kumar, S. Nitrogen use efficiency reimagined: Advancements in agronomic, ecophysiological, and molecular strategies. J. Plant Nutr. 2024, 1–27. [Google Scholar] [CrossRef]
- Llamas, A.; Leon-Miranda, E.; Tejada-Jimenez, M. Microalgal and nitrogen-fixing bacterial consortia: From interaction to biotechnological potential. Plants 2023, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Masmoudi, K. Molecular Breakthroughs in Modern Plant Breeding Techniques. Hortic. Plant J. 2024, 11, 15–41. [Google Scholar]
- Lorenzi, A.S.; Chia, M.A. Cyanobacteria’s power trio: Auxin, siderophores, and nitrogen fixation to foster thriving agriculture. World J. Microbiol. Biotechnol. 2024, 40, 1–18. [Google Scholar]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar]
- Nur, M.M.A.; Murni, S.W.; Setyoningrum, T.M.; Hadi, F.; Widayati, T.W.; Jaya, D.; Sulistyawati, R.R.E.; Puspitaningrum, D.A.; Dewi, R.N.; Hasanuzzaman, M. Innovative strategies for utilizing microalgae as dual-purpose biofertilizers and phycoremediators in agroecosystems. Biotechnol. Rep. 2024, 45, e00870. [Google Scholar]
- Zayadan, B.; Matorin, D.; Baimakhanova, G.; Bolathan, K.; Oraz, G.; Sadanov, A. Promising microbial consortia for producing biofertilizers for rice fields. Microbiology 2014, 83, 391–397. [Google Scholar]
- Prasanna, R.; Babu, S.; Bidyarani, N.; Kumar, A.; Triveni, S.; Monga, D.; Mukherjee, A.K.; Kranthi, S.; Gokte-Narkhedkar, N.; Adak, A. Prospecting cyanobacteria-fortified composts as plant growth promoting and biocontrol agents in cotton. Exp. Agric. 2015, 51, 42–65. [Google Scholar]
- Ali, M.A.; Sattar, M.; Islam, M.N.; Inubushi, K. Integrated effects of organic, inorganic and biological amendments on methane emission, soil quality and rice productivity in irrigated paddy ecosystem of Bangladesh: Field study of two consecutive rice growing seasons. Plant Soil 2014, 378, 239–252. [Google Scholar]
- Mishra, A.; Rajput, S.; Gupta, P.S.; Goyal, V.; Singh, S.; Sharma, S.; Shukla, S.; Singh, A.; Shukla, K.; Varma, A. Role of Cyanobacteria in Rhizospheric Nitrogen Fixation. In Soil Nitrogen Ecology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 497–519. [Google Scholar]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar]
- Yang, T.; Siddique, K.H.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Du, C.; Li, G.; Xia, R.; Li, C.; Zhu, Q.; Li, X.; Li, J.; Zhao, C.; Tian, Z.; Zhang, L. New insights into cyanobacterial blooms and the response of associated microbial communities in freshwater ecosystems. Environ. Pollut. 2022, 309, 119781. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Schulte, R.; Creamer, R.; Delgado, A.; Van Leeuwen, J.; Lehtinen, T.; Rutgers, M.; Spiegel, H.; Staes, J.; Tóth, G. The elusive role of soil quality in nutrient cycling: A review. Soil Use Manag. 2016, 32, 476–486. [Google Scholar] [CrossRef]
- Carvajal-Muñoz, J.; Carmona-Garcia, C. Benefits and limitations of biofertilization in agricultural practices. Livest. Res. Rural. Dev. 2012, 24, 1–8. [Google Scholar]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Xiong, H.; Li, Y.; Zhang, Y.; Huang, X.; Yang, Y.; Zhu, H.; Jiang, T. Influence of long-term fertilization on soil aggregates stability and organic carbon occurrence characteristics in karst yellow soil of Southwest China. Front. Plant Sci. 2023, 14, 1126150. [Google Scholar] [CrossRef]
- Shi, Y.; Niu, X.; Chen, B.; Pu, S.; Ma, H.; Li, P.; Feng, G.; Ma, X. Chemical fertilizer reduction combined with organic fertilizer affects the soil microbial community and diversity and yield of cotton. Front. Microbiol. 2023, 14, 1295722. [Google Scholar] [CrossRef]
- Si, S.; Wu, Y.; Li, Y.; Yang, S.; Peng, X.; Luo, Y. Divergent soil health responses to long-term inorganic and organic fertilization management on subtropical upland red soil in China. Ecol. Indic. 2023, 154, 110486. [Google Scholar]
- Huang, S.; Rui, W.; Peng, X.; Huang, Q.; Zhang, W. Organic carbon fractions affected by long-term fertilization in a subtropical paddy soil. Nutr. Cycl. Agroecosystems 2010, 86, 153–160. [Google Scholar] [CrossRef]
- Aumtong, S.; Chotamonsak, C.; Pongwongkam, P.; Cantiya, K. Chemical Fertilization Alters Soil Carbon in Paddy Soil through the Interaction of Labile Organic Carbon and Phosphorus Fractions. Agronomy 2023, 13, 1588. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function: A field study with sweet corn. Biol. Fertil. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. Sustain. Agric. 2011, 2, 761–786. [Google Scholar]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A. Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)]. Bioresour. Technol. 2010, 101, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Udovic, M.; McBride, M.B. Influence of compost addition on lead and arsenic bioavailability in reclaimed orchard soil assessed using Porcellio scaber bioaccumulation test. J. Hazard. Mater. 2012, 205, 144–149. [Google Scholar] [CrossRef]
- Tandy, S.; Healey, J.; Nason, M.; Williamson, J.; Jones, D. Heavy metal fractionation during the co-composting of biosolids, deinking paper fibre and green waste. Bioresour. Technol. 2009, 100, 4220–4226. [Google Scholar] [CrossRef]
- Iqbal, J.; Hu, R.; Lin, S.; Hatano, R.; Feng, M.; Lu, L.; Ahamadou, B.; Du, L. CO2 emission in a subtropical red paddy soil (Ultisol) as affected by straw and N-fertilizer applications: A case study in Southern China. Agric. Ecosyst. Environ. 2009, 131, 292–302. [Google Scholar] [CrossRef]
- Meijide, A.; Cárdenas, L.M.; Sánchez-Martín, L.; Vallejo, A. Carbon dioxide and methane fluxes from a barley field amended with organic fertilizers under Mediterranean climatic conditions. Plant Soil 2010, 328, 353–367. [Google Scholar] [CrossRef]
- Rabalais, N.N. Nitrogen in aquatic ecosystems. AMBIO A J. Hum. Environ. 2002, 31, 102–112. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, S. Soil Structure and Plant Growth. In Soil Physical Environment and Plant Growth: Evaluation and Management; Springer: Berlin/Heidelberg, Germany, 2023; pp. 125–154. [Google Scholar]
- Guhra, T.; Stolze, K.; Totsche, K.U. Pathways of biogenically excreted organic matter into soil aggregates. Soil Biol. Biochem. 2022, 164, 108483. [Google Scholar] [CrossRef]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Auty, K.M.; Zaman, R.U.; Kamal, N.N. Innovative Approaches to Stability of Embankment—A Role of Chemical Stabilization in Erosion Mitigation. Bachelor’s thesis, Department of Civil and Environmental Engineering (CEE), Islamic University, Board Bazar, Bangladesh, 2024. [Google Scholar]
- Wang, N.; Tian, S.; Gu, L.; Xu, L.; Qiu, Y.; Van Den Top, T.; Gonzalez-Hernandez, J.L.; Hildreth, M.B.; Li, S.; Zhou, R. Isolation of Potential Photosynthetic N 2-Fixing Microbes from Topsoil of Native Grasslands in South Dakota. In Proceedings of the South Dakota Academy of Science; South Dakota State University: Brookings, SD, USA, 2018; p. 117. [Google Scholar]
- Wang, X. Managing land carrying capacity: Key to achieving sustainable production systems for food security. Land 2022, 11, 484. [Google Scholar] [CrossRef]
- Sun, N.; Sarkar, B.; Li, S.; Tian, Y.; Sha, L.; Gao, Y.; Luo, X.; Yang, X. Biochar Addition Increased Soil Carbon Storage but Did Not Exacerbate Soil Carbon Emission in Young Subtropical Plantation Forest. Forests 2024, 15, 917. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Gong, J.; Zhang, L.; Jiang, Q.; Huang, K.; Ding, W. Soil conditions on bacterial wilt disease affect bacterial and fungal assemblage in the rhizosphere. AMB Express 2022, 12, 110. [Google Scholar] [PubMed]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.-R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef]
- Rigobelo, E. Symbiosis in Nature; BoD–Books on Demand: Norderstedt, Germany, 2023. [Google Scholar]
- Srivastav, A.L.; Dhyani, R.; Ranjan, M.; Madhav, S.; Sillanpää, M. Climate-resilient strategies for sustainable management of water resources and agriculture. Environ. Sci. Pollut. Res. 2021, 28, 41576–41595. [Google Scholar]
- Kučera, A.; Samec, P.; Bajer, A.; Skene, K.R.; Vichta, T.; Vranová, V.; Meena, R.S.; Datta, R. Forest Soil Water in Landscape Context; IntechOpen: London, UK, 2020; Volume 45. [Google Scholar]
- Rashid, M.; Kamruzzaman, M.; Haque, A.; Krehenbrink, M. Soil microbes for sustainable agriculture. In Sustainable Management of Soil and Environment; Springer: Singapore, 2019; pp. 339–382. [Google Scholar]
- Kumar, M.; Singh, D.; Prabha, R.; Sharma, A.K. Role of cyanobacteria in nutrient cycle and use efficiency in the soil. In Nutrient Use Efficiency: From Basics to Advances; Springer: New Delhi, India, 2015; pp. 163–171. [Google Scholar]
- Lal, R. Reducing carbon footprints of agriculture and food systems. Carbon Footpr. 2022, 1, 3. [Google Scholar]
- De La Hoz, A.L.A. Understanding Cyanobacteria-Based Biofertilizers in Soil-Water and Soil-Plant Systems. Ph.D. thesis, University of Minnesota, Minneapolis, MN, USA, 2021. [Google Scholar]
- Sharma, G.K.; Khan, S.A.; Shrivastava, M.; Bhattacharyya, R.; Sharma, A.; Gupta, N.; Bhatia, A. Phycoremediated N-fertilization approaches on reducing environmental impacts of agricultural nitrate leaching. J. Clean. Prod. 2022, 345, 131120. [Google Scholar]
- Maqubela, M.; Mnkeni, P.; Issa, O.M.; Pardo, M.; D’acqui, L. Nostoc cyanobacterial inoculation in South African agricultural soils enhances soil structure, fertility, and maize growth. Plant Soil 2009, 315, 79–92. [Google Scholar]
- Do Nascimento, M.; Battaglia, M.E.; Rizza, L.S.; Ambrosio, R.; Di Palma, A.A.; Curatti, L. Prospects of using biomass of N2-fixing cyanobacteria as an organic fertilizer and soil conditioner. Algal Res. 2019, 43, 101652. [Google Scholar]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar]
- Nawaz, T.; Nelson, D.; Fahad, S.; Saud, S.; Aaqil, M.; Adnan, M.; Saleem, A.; Bibi, M.; Joshi, N.; Rahman, T.U. Impact of elevated CO2 and temperature on overall agricultural productivity. In Challenges and Solutions of Climate Impact on Agriculture; Elsevier: Amsterdam, The Netherlands, 2025; pp. 163–202. [Google Scholar]
- Issa, A.A.; Abd-Alla, M.H.; Ohyama, T. Nitrogen fixing cyanobacteria: Future prospect. Adv. Biol. Ecol. Nitrogen Fixat. 2014, 2, 24–48. [Google Scholar]
- Björn, L.O.; Govindjee. The evolution of photosynthesis and its environmental impact. In Photobiology; Springer: New York, NY, USA, 2015; pp. 207–230. [Google Scholar]
- Bennett, E.M.; Murray, J.W.; Isalan, M. Engineering nitrogenases for synthetic nitrogen fixation: From pathway engineering to directed evolution. BioDesign Res. 2023, 5, 0005. [Google Scholar]
- Saier, M.H.; Jacobson, G.R. The Molecular Basis of Sex and Differentiation: A Comparative Study of Evolution, Mechanism and Control in Microorganisms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kufryk, G. Exploring ecological diversity and biosynthetic potential of cyanobacteria for biofuel production. In Cyanobacterial Lifestyle and its Applications in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 197–230. [Google Scholar]
- Braselton, N. Synthetic Biology for Autotrophic and Heterotrophic Production of Ethanol; South Dakota State University: Brookings, SD, USA, 2016. [Google Scholar]
- Khetkorn, W.; Khanna, N.; Incharoensakdi, A.; Lindblad, P. Metabolic and genetic engineering of cyanobacteria for enhanced hydrogen production. Biofuels 2013, 4, 535–561. [Google Scholar]
- Sengupta, A.; Pakrasi, H.B.; Wangikar, P.P. Recent advances in synthetic biology of cyanobacteria. Appl. Microbiol. Biotechnol. 2018, 102, 5457–5471. [Google Scholar]
- Lery, L.M.; Bitar, M.; Costa, M.G.; Rössle, S.C.; Bisch, P.M. Unraveling the molecular mechanisms of nitrogenase conformational protection against oxygen in diazotrophic bacteria. BMC Genom. 2010, 11, 1–11. [Google Scholar]
- Luan, G.; Lu, X. Tailoring cyanobacterial cell factory for improved industrial properties. Biotechnol. Adv. 2018, 36, 430–442. [Google Scholar]
- Priyadarshini, P.; Choudhury, S.; Tilgam, J.; Bharati, A.; Sreeshma, N. Nitrogen fixing cereal: A rising hero towards meeting food security. Plant Physiol. Biochem. 2021, 167, 912–920. [Google Scholar]
- Alexander, M. Ecological constraints on nitrogen fixation in agricultural ecosystems. In Advances in Microbial Ecology; Springer: Berlin/Heidelberg, Germany, 1985; Volume 8, pp. 163–183. [Google Scholar]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Hillebrand, H.; Harpole, W.; Gross, K.; Ptacnik, R. Separating the influence of resource ‘availability’from resource ‘imbalance’on productivity–diversity relationships. Ecol. Lett. 2009, 12, 475–487. [Google Scholar]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Q.; Huang, H. Current states and challenges of salt-affected soil remediation by cyanobacteria. Sci. Total Environ. 2019, 669, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, M.W.; Makhalanyane, T.P.; Cowan, D.A.; Valverde, A. Cyanobacteria and Alphaproteobacteria may facilitate cooperative interactions in niche communities. Front. Microbiol. 2017, 8, 2099. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Herve, V.; Labbe, J.; Lastovetsky, O.A. Bacterial–fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef]
- Wen-Hui, Z.; Zu-Cong, C. Effect of soil management practices and environmental factors on soil microbial diversity: A review. Biodivers. Sci. 2004, 12, 456. [Google Scholar]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Zdarta, J.; Nga, T.T.; Nghiem, L.D. Blue-green algae in surface water: Problems and opportunities. Curr. Pollut. Rep. 2020, 6, 105–122. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Peng, C.; Li, D. Replacing nitrogen fertilizer with nitrogen-fixing cyanobacteria reduced nitrogen leaching in red soil paddy fields. Agric. Ecosyst. Environ. 2021, 312, 107320. [Google Scholar] [CrossRef]
- Sarma, M.K.; Kaushik, S.; Goswami, P. Cyanobacteria: A metabolic power house for harvesting solar energy to produce bio-electricity and biofuels. Biomass Bioenergy 2016, 90, 187–201. [Google Scholar] [CrossRef]
- Guo, K.; Yang, J.; Yu, N.; Luo, L.; Wang, E. Biological nitrogen fixation in cereal crops: Progress, strategies, and perspectives. Plant Commun. 2023, 4, 100499. [Google Scholar] [PubMed]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering rhizobacteria for sustainable agriculture. ISME J. 2021, 15, 949–964. [Google Scholar] [PubMed]
- Behler, J.; Vijay, D.; Hess, W.R.; Akhtar, M.K. CRISPR-based technologies for metabolic engineering in cyanobacteria. Trends Biotechnol. 2018, 36, 996–1010. [Google Scholar] [PubMed]
- Patel, V.K.; Das, A.; Kumari, R.; Kajla, S. Recent progress and challenges in CRISPR-Cas9 engineered algae and cyanobacteria. Algal Res. 2023, 71, 103068. [Google Scholar]
- Hwang, J.; Ye, D.-Y.; Jung, G.Y.; Jang, S. Mobile genetic element-based gene editing and genome engineering: Recent advances and applications. Biotechnol. Adv. 2024, 72, 108343. [Google Scholar]
- Sarker, N.K.; Kaparaju, P. Microalgal Bioeconomy: A Green Economy Approach Towards Achieving Sustainable Development Goals. Sustainability 2024, 16, 11218. [Google Scholar] [CrossRef]
- Plá, C.L.; Cobos-Porras, L. Salinity: Physiological impacts on legume nitrogen fixation. In Legume Nitrogen Fixation in a Changing Environment; Springer: Cham, Switzerland, 2015; pp. 35–65. [Google Scholar]
- Mishra, A.K.; Kaushik, M.S.; Tiwari, D. Nitrogenase and hydrogenase: Enzymes for nitrogen fixation and hydrogen production in cyanobacteria. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2019; pp. 173–191. [Google Scholar]
- Cui, J.; Sun, H.; Chen, R.; Sun, J.; Mo, G.; Luan, G.; Lu, X. Multiple routes toward engineering efficient cyanobacterial photosynthetic biomanufacturing technologies. Green Carbon 2023, 1, 210–226. [Google Scholar]
- Zhang, W.; Song, X. Synthetic Biology of Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1080. [Google Scholar]
- Giller, K.E.; Cadisch, G. Future benefits from biological nitrogen fixation: An ecological approach to agriculture. In Proceedings of the Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems: Extended Versions of Papers Presented at the Symposium on Biological Nitrogen Fixation for Sustainable Agriculture at the 15th Congress of Soil Science, Acapulco, Mexico, 10–16 July 1994; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 255–277. [Google Scholar]
- Ambrosio, R.; Rizza, L.S.; Do Nascimento, M.; Pacheco, H.G.J.; Ramos, L.M.M.; Hernandez, J.A.; Curatti, L. Promises and challenges for expanding the use of N2-fixing cyanobacteria as a fertilizer for sustainable agriculture. In Cyanobacterial Lifestyle and its Applications in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 99–158. [Google Scholar]
- Bhuyan, P.P.; Nayak, R.; Jena, M.; Pradhan, B. Convoluted role of cyanobacteria as biofertilizer: An insight of sustainable agriculture. Vegetos 2023, 36, 309–321. [Google Scholar]
- Garwi, J.; Masengu, R.; Chiwaridzo, O.T. Emerging Technologies and Marketing Strategies for Sustainable Agriculture; IGI Global: Hershey, PA, USA, 2024. [Google Scholar]
- Saud, S.; Nawaz, T.; Hassan, S.; Ur Rahman, T.; Rasheed, M.N.; Hussain, S.; Fahad, S. Nitrogen-fixing cyanobacteria and soil enrichment for a greener future. In Environment, Climate, Plant and Vegetation Growth; Springer: Berlin/Heidelberg, Germany, 2024; pp. 391–424. [Google Scholar]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Bleakley, B.; Zhou, R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules 2024, 29, 2534. [Google Scholar] [CrossRef]
- Sebesta, J.; Xiong, W.; Guarnieri, M.T.; Yu, J. Biocontainment of genetically engineered algae. Front. Plant Sci. 2022, 13, 839446. [Google Scholar]
- Rocha, F.; Esteban Lucas-Borja, M.; Pereira, P.; Muñoz-Rojas, M. Cyanobacteria as a nature-based biotechnological tool for restoring salt-affected soils. Agronomy 2020, 10, 1321. [Google Scholar] [CrossRef]
- Strickler, D. The Complete Guide to Restoring Your Soil: Improve Water Retention and Infiltration; Support Microorganisms and Other Soil Life; Capture More Sunlight; and Build Better Soil with No-Till, Cover Crops, and Carbon-Based Soil Amendments. Storey Publishing: Adams, MA, USA, 2021. [Google Scholar]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Granjou, C.; Phillips, C. Living and labouring soils: Metagenomic ecology and a new agricultural revolution? BioSocieties 2019, 14, 393–415. [Google Scholar] [CrossRef]
- Zhang, X.; Ward, B.B.; Sigman, D.M. Global nitrogen cycle: Critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem. Rev. 2020, 120, 5308–5351. [Google Scholar] [CrossRef]
- Pereira, I.; Rangel, A.; Chagas, B.; de Moura, B.; Urbano, S.; Sassi, R.; Camara, F.; Castro, C. Microalgae growth under mixotrophic condition using agro-Industrial waste: A review. In Biotechnological Applications of Biomass; IntechOpen: London, UK, 2021; Volume 10. [Google Scholar]
- Omar, A.; Almomani, F.; Qiblawey, H.; Rasool, K. Advances in Nitrogen-Rich Wastewater Treatment: A Comprehensive Review of Modern Technologies. Sustainability 2024, 16, 2112. [Google Scholar] [CrossRef]
| Soil Characteristics | Mechanism | Types of Organic Fertilizers | References |
|---|---|---|---|
| Soil physical properties | Reduce soil bulk density, increase total porosity, increase the number and stability of soil aggregates, enhance the ability of soil to retain water and fertilizer, and alleviate soil acidification. | Livestock manure, farm manure, crop straw, biological waste, green fertilizer, commercial organic fertilizer | [130,131,132,133] |
| Soil nutrient | Improve the capacity of soil fertilizer supply; Accelerate the activation rate of humic acid to soil nutrients, improve the activities of microorganisms and enzymes related to nutrient conversion; maintain the balance of available nutrient supply and improve fertilizer utilization efficiency; increase the availability of trace elements. | Livestock manure, farm manure, crop straw, biological waste, sludge, green manure, commercial organic fertilizer | [134,135,136] |
| Soil microorganism | Increase organic matter and soil fertility, provide carbon source, nitrogen source, energy and binding site for soil microorganisms and enzymes; improve the soil microecological environment and promote the growth and reproduction of microorganisms. | Livestock manure, farm manure, crop straw, biological waste, sludge, green manure, commercial organic fertilizer | [137,138] |
| Soil heavy metals | They carry high levels of heavy metals; the availability of heavy metals was affected by changing the physical and chemical properties of soil such as pH, SOM, and Eh. The availability of heavy metals depends on the adsorption and desorption processes. | Livestock manure, sludge, commercial organic fertilizer | [139,140] |
| Soil greenhouse gas | Release more CO2 by increasing soil organic matter and total porosity and soil respiration; provide an abundant methanogenic matrix and suitable environment for methanogenic bacteria to grow and release more CH4; by changing soil C/N, the formation and emission of nitrification and denitrification reaction products of N2O are affected, and different organic fertilizers show uncertainty. | Livestock manure, farm manure, crop straw, biological waste, commercial organic fertilizer | [141,142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, T.; Fahad, S.; Gu, L.; Xu, L.; Zhou, R. Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security. Nitrogen 2025, 6, 16. https://doi.org/10.3390/nitrogen6010016
Nawaz T, Fahad S, Gu L, Xu L, Zhou R. Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security. Nitrogen. 2025; 6(1):16. https://doi.org/10.3390/nitrogen6010016
Chicago/Turabian StyleNawaz, Taufiq, Shah Fahad, Liping Gu, Lan Xu, and Ruanbao Zhou. 2025. "Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security" Nitrogen 6, no. 1: 16. https://doi.org/10.3390/nitrogen6010016
APA StyleNawaz, T., Fahad, S., Gu, L., Xu, L., & Zhou, R. (2025). Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security. Nitrogen, 6(1), 16. https://doi.org/10.3390/nitrogen6010016








