Abstract
With the objective of water analysis, a microsystem was developed, by using Resazurin molecule as an indirect bio sensor. The detection principle consists to monitor optically the oxygen consumption of E. Coli bacteria towards their metabolism in presence of organic pollutants. Thus, aiming on Glucose detection, a concentration-dependent inhibition effect on oxygen consumption rate was evidenced in the [0–0.41 mM] range of organic pollutants.
1. Introduction
Assessment of water quality has become an important environmental issue during the last years. Industrial waste is known to have a severe impact on living microorganisms. One of the key parameters that translate the quality of water is the ‘BOD’ (biochemical oxygen demand) parameter. Its value is based on the evaluation of the dissolved oxygen used by microorganisms as bacteria to degrade the organic pollutants [1]. It is therefore a major challenge to monitor their different quantities. Normalized methods are currently performed with heavy equipment in dedicated laboratories [2]. Therefore it is important to develop a new approach based on microsystems technologies that allows a rapid feedback at lower cost. Biosensors and miniaturized microsystems have already demonstrated a wide set of advantages [3,4]. In this study, a Glass/PDMS/Resazurin-based chip has been developed to monitor bacteria metabolism, detect organic pollutants and indirectly conclude about oxygen rate ‘bacteria consumption. The complete system is planned to integrate eight microwells having different types of bacteria, and connected to an optical measurement system.
2. Biosensors and Experimental Protocol
2.1. Principles
The viability of our bacteria in presence of different Glucose concentrations is estimated through the use of a Resazurin Blue dye [5,6,7]. It reflects the bacteria respiration related to organic pollutants degradation; the process can be quantified by evaluating the emitted fluorescence signal.
The Resazurin is reduces by diaphorase in presence of NADH to a highly fluorescent molecule “Resorufin” (Equation (1)).
Resazurin is an electron acceptor in the biodegradation reaction of an organic molecule, such as Glucose in the Equation (2).
2.2. Experimental Protocol
A colony of E. Coli bacteria was grown aerobically with stirring at 37 °C during 12 h in 5 cm3 Luria Bertani (LB) medium. The bacteria population is controlled and adjusted by optical density (measured by a Fisherbrand-Digital colorimeter). A second bacteria culture has started for two hours; the optical density of bacteria was adjusted to be 0.25: it corresponds to a concentration of 2 × 108 bact/mL. After culture, bacteria were washed three times with magnesium sulphate (MgSO4). The E. Coli strains were maintained in an M9 medium with Resazurin and different Glucose concentrations mimicking organic pollutants; it is injected with a pipet in the microfluidic chambers prior to measurement.
2.3. Design and Technological Biochip Process
As depicted in Figure 1, the biosensor was designed as an Eight-well chip. Chambers were filled up with the water sample mixed with the solution containing bacteria. The chip was then connected to measurement system for optical readout.
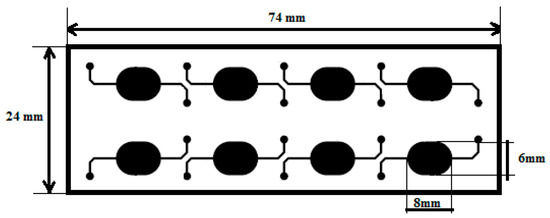
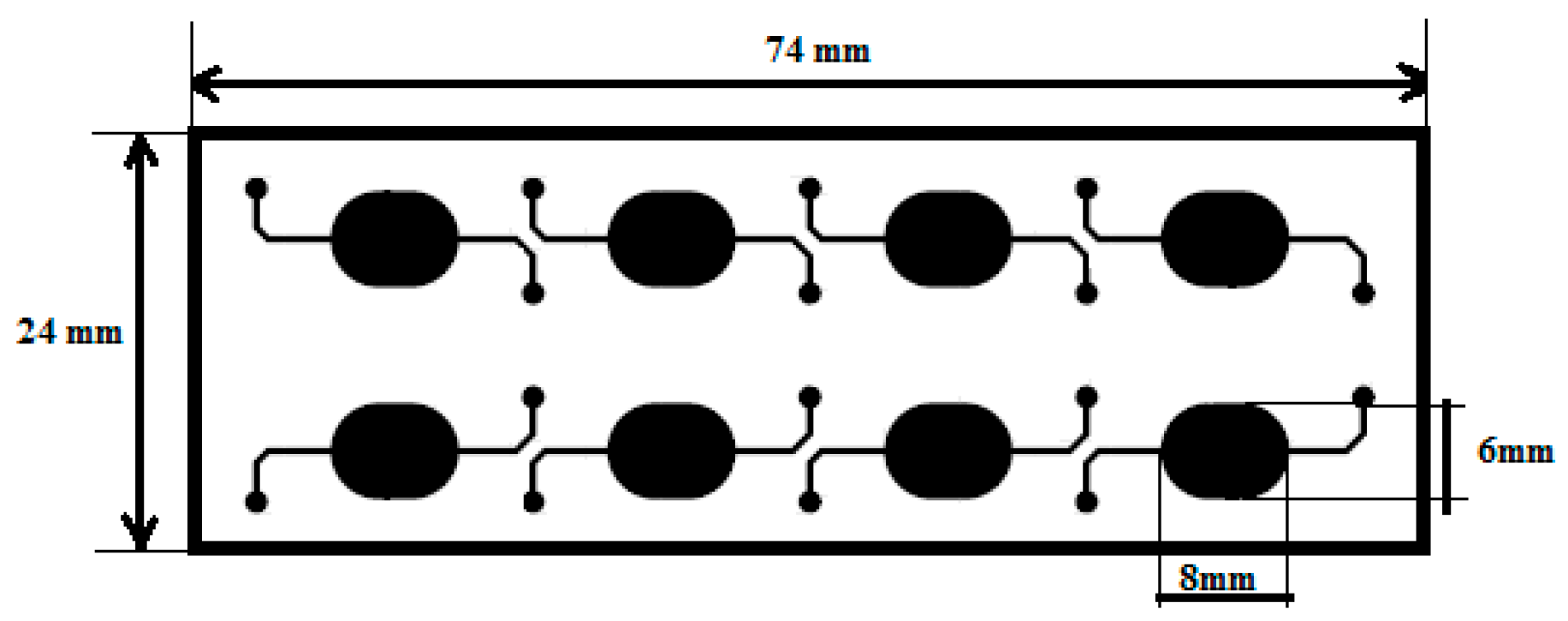
Figure 1.
Top view of the microfluidic chip design [8].
The devices were fabricated by using PDMS and Glass materials: this choice is based on technological feasibility and biocompatibility; it is also complies with optical detection by its low auto fluorescence.
The microfluidic chip is 24 mm wide and 74 mm long and contains 8 oval shaped 24 μL wells. Micro chambers dimensions were specified according to the optical system requirements.
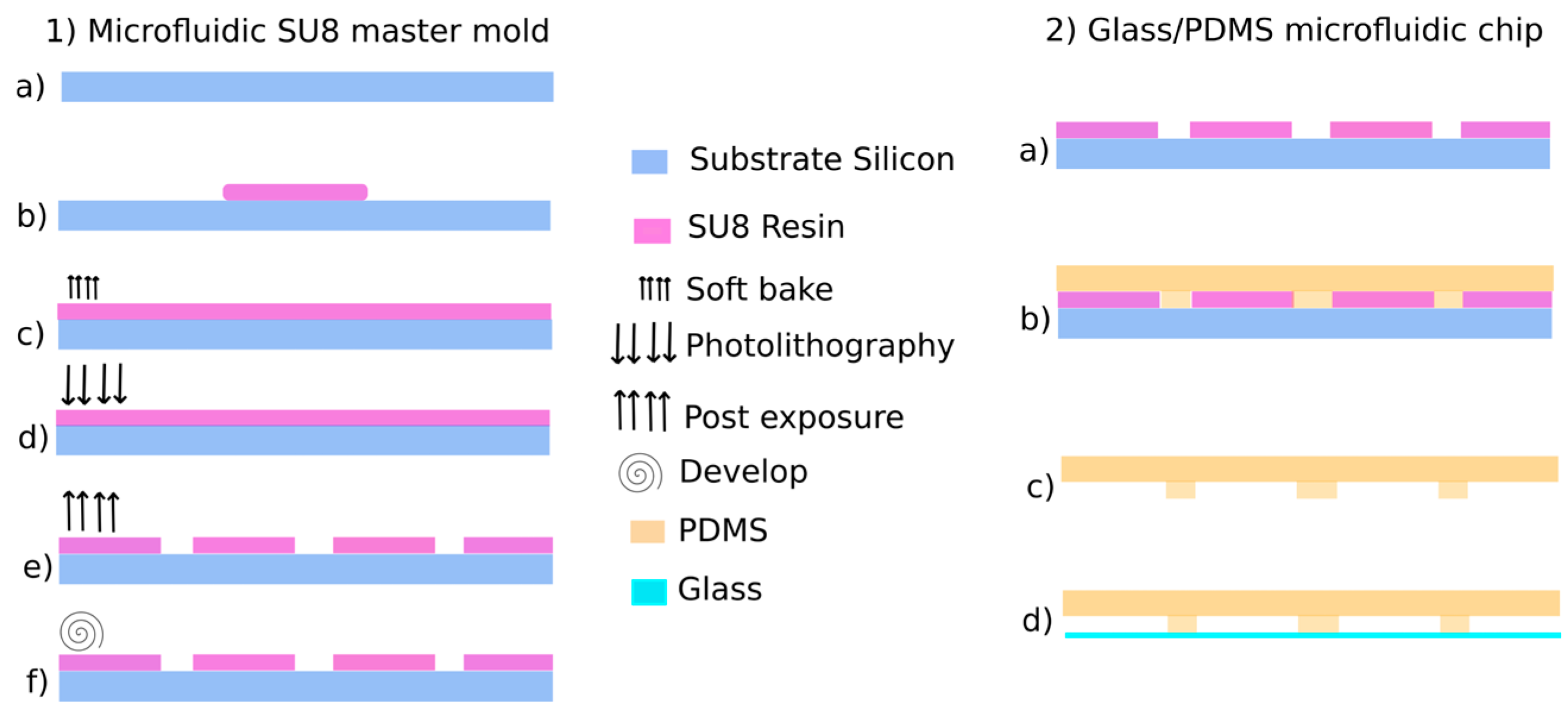
The manufacturing process is detailed in Figure 2. Firstly, a SU8 mold was obtained by pattering a 500 μm-thick SU8 photoresist layer. The PDMS microfluidic chip was then molded following a standard soft lithography process and bonded to a glass substrate.
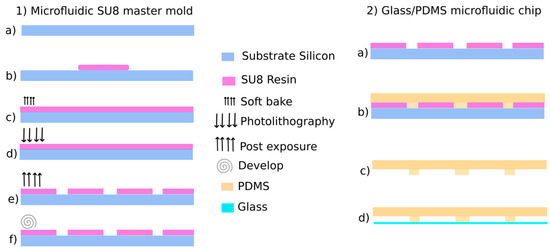
Figure 2.
GlassPDMS biochip process: (1) Microfluidic SU8 master mold and (2) Glass/PDMS microfluidic chip [8].
2.4. Measurement System
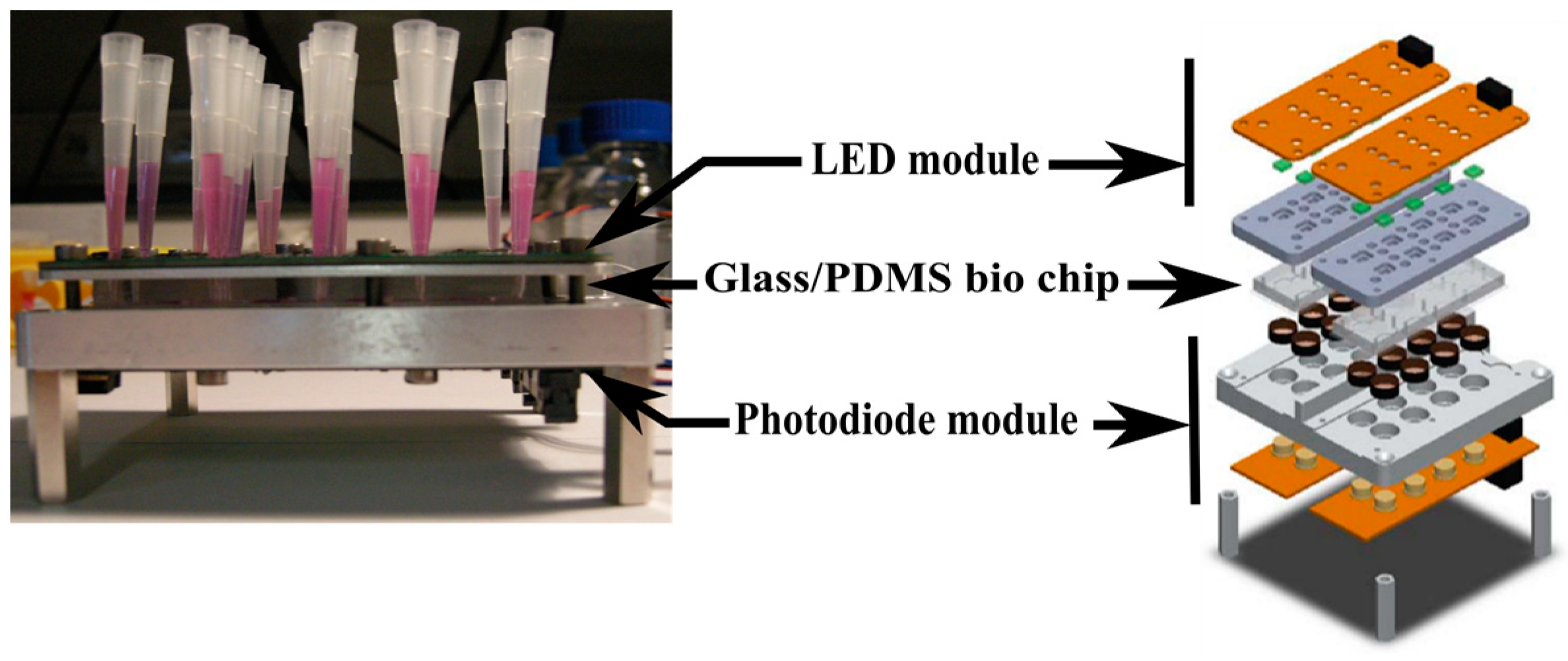
The optical readout setup is shown in Figure 3 [8]. A LED module (OSRAM LTN91E) is used to excite the Resazurin at 560 nm and a photodiode module (Texas Instrument OPT301) is used to detect Resorufin fluorescence. This latter one is connected to a data acquisition system and a network analyzer to collect data. Experiments were performed in a dark room at 30 °C.
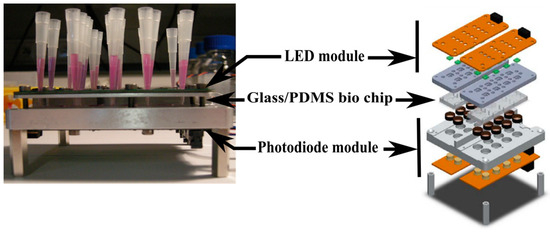
Figure 3.
Fluorescence readout setup [8].
3. Results and Discussion
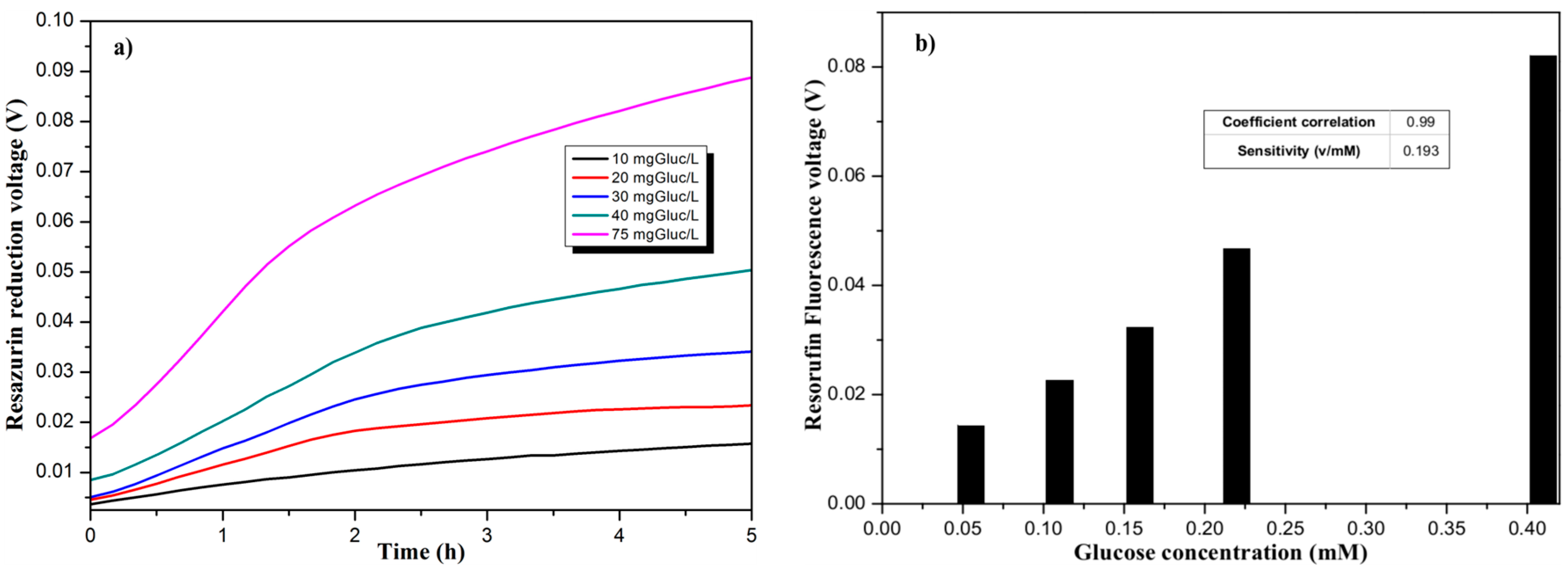
Measurements were done over five hours with a periodicity of 10 min, with 20 mA as LED excitation current. The fluorescence integration time has been set at 100 ms. As explained earlier, the variation of fluorescence signal illustrates the respiratory activity of bacteria. The curves presented in Figure 4a show the difference in signal fluorescence related to glucose concentration.
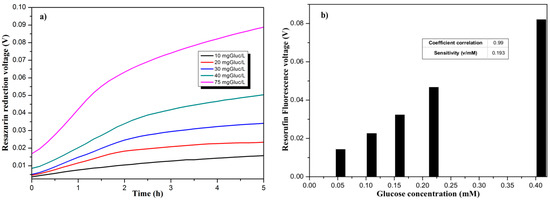
Figure 4.
(a) Temporal evolution of the Resazurin reduction for different glucose concentrations; (b) Evaluation of the Resorufin fluorescence for different glucose concentrations.
It can be observed that for the first 90 min, the signal is progressing with different rates related to Glucose concentrations. After two hours of measurement, a plateau is reached where the impact of Glucose can be clearly observed. Over 75 mg/L of Glucose concentration, the signal is saturated. As presented in Figure 4b, our sensor has a sensitivity of 0.193 V/mM.
4. Conclusions
In this work, a microsystem approach has been used to detect different glucose concentrations, mimicking hence organic pollutants.
Despite partial uncertainties related to the porosity of the PDMS when the measurement duration is longer than three hours, we have demonstrated that this approach is suitable for a concentration range of organic carbon varying from 10 to 75 mg/L with a sensitivity of 0.193 V/mM. These first results demonstrate that the proposed chip biosensor can follows the change in respiratory activity induced by organic pollutants and reflected through a modification in oxygen consumption rate. It can be therefore an efficient indicator of water quality.
Nevertheless, further studies should be conducted to still improve detection properties while optimizing the oxygen diffusion, material of chip and measures on site and online.
Acknowledgments
This work was partly supported by LAAS-CNRS micro and nanotechnologies platform member of the French RENATECH network.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ISO 2003 Water Quality-Determination of Biological Oxygen Demand after n Days (BOD ISO 5815-1:2003). Water Qual. 2003, 2003, 5815-1.
- Association APHA. A Standard Method for the Examination of Water and Wastewater, 16th ed.; American Public Health Association: Washington, DC, USA, 1986. [Google Scholar]
- Jang, A.; Zou, Z.; Lee, K.K.; Ahn, C.H.; Bishop, P.L. State-of-the-art lab chip sensors for environmental water monitoring. J. Meas. Sci. Technol. 2011, 18, 032001. [Google Scholar] [CrossRef]
- Jouaneau, S.; Recoules, L.; Durand, M.J.; Boukabache, A.; Picot, V.; Primault, Y.; Lakel, A.; Sengelin, M.; Barillon, B.; Thouand, G. Methods for assessing biochemical oxygen demand (bod): A review. Water Res. 2011, 49, 62–82. [Google Scholar] [CrossRef]
- McNicholla, B.P.; Mcgrath, J.W.; Quinn, J.P. Development and application of a resazurin-based biomass activity test for activated sludge plant management. Water Res. 2007, 41, 127–133. [Google Scholar] [CrossRef]
- Lambourne, S.R.; Gibb, Z.; Aitken, R.J. The resazurin reduction assay: A diagnostic tool for Thoroughbred breeders. J. Equine Vet. Sci. 2014, 34, 46. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, Y.; Sin, M.L.Y.; Mach, K.E.; Zhang, D.D.; Gau, V.; Liao, J.C.; Wong, P.K. Rapid antimicrobial susceptibility testing using high surface-to-volume ratio microchannels. Natl. Inst. Health 2010, 82, 1012. [Google Scholar]
- Recoules, L. Biocapteur Pour la Surveillance de la Qualité de l’eau: Application Aux Eaux Pluviales et de Stations D’épurations. Ph.D. Thesis, University of Toulouse, Toulouse, France, 2015. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).