Exploring Schiff Bases as Promising Alternatives to Traditional Drugs in the In Silico Treatment of Anti-Leishmaniasis as Trypanothione Reductase Inhibitors †
Abstract
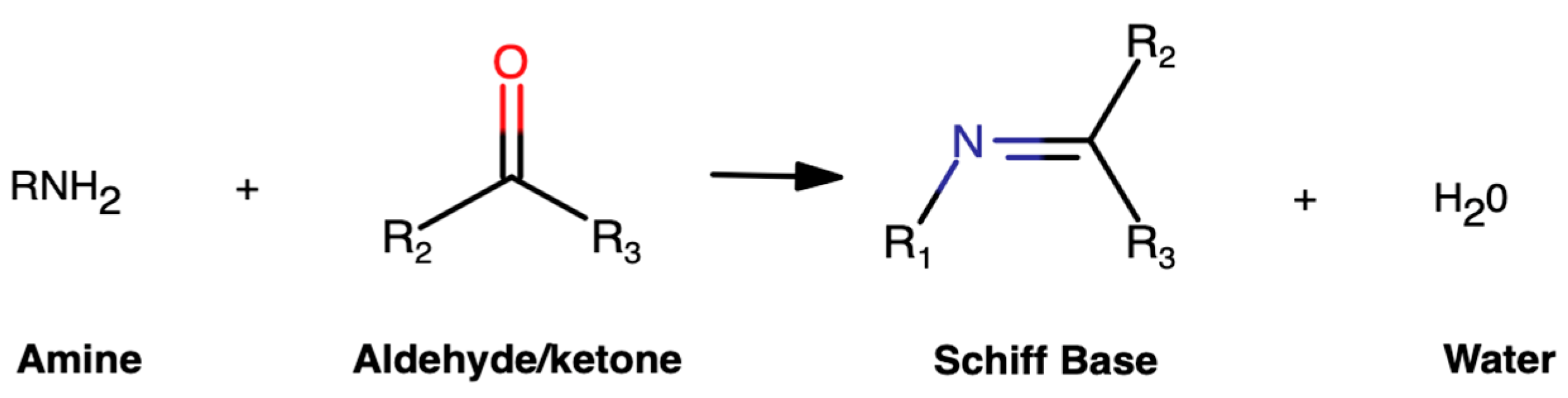
:1. Introduction
2. Methods
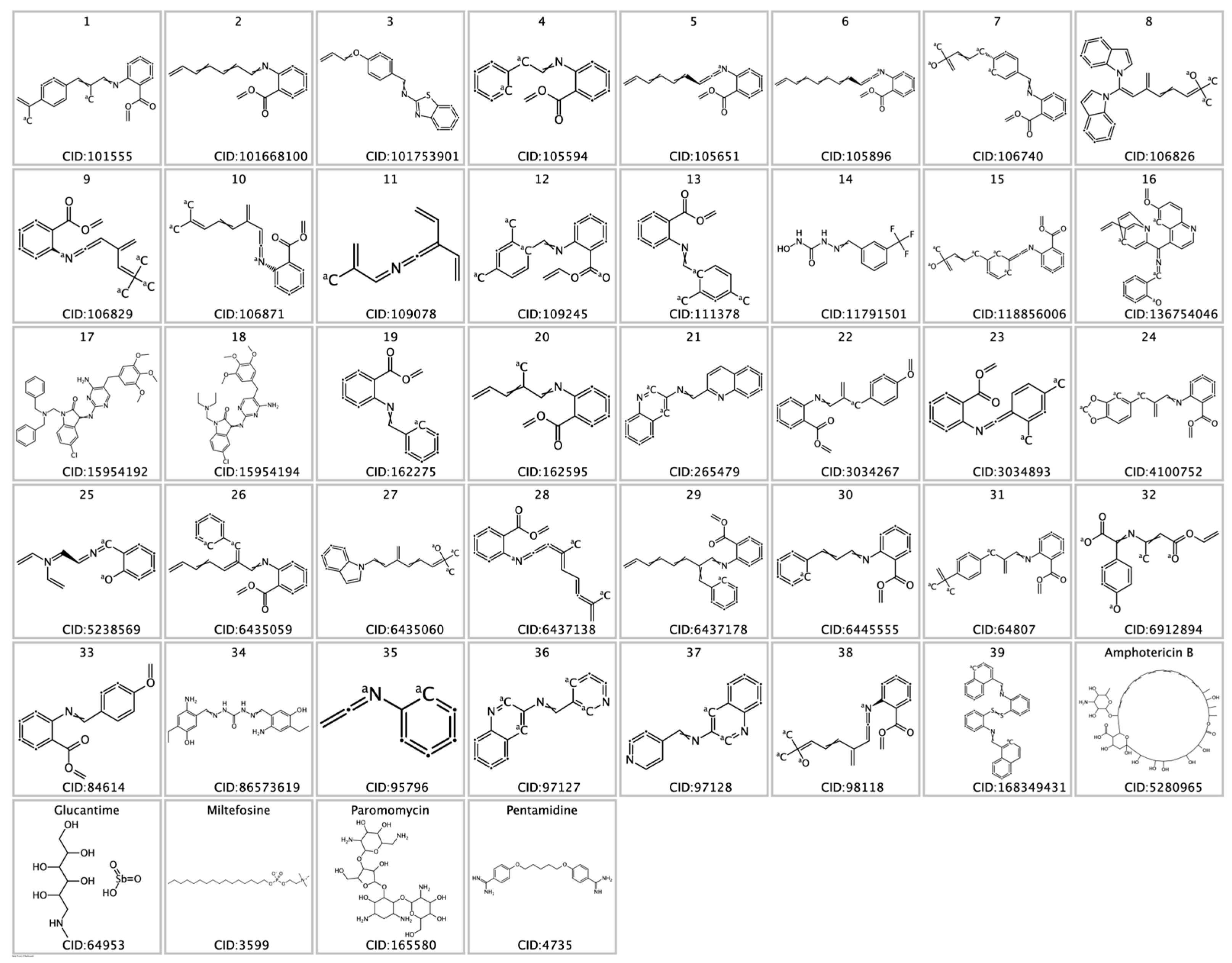
2.1. Data Preparation for Virtual Screening
2.2. Protein–Ligand Interaction Analysis
3. Results and Discussion
Virtual Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489. [Google Scholar] [CrossRef] [PubMed]
- Arenas, R.; Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J. Leishmaniasis: A Review. F1000Res 2017, 6, 750. [Google Scholar] [CrossRef]
- Rath, S.; Augusto Trivelin, L.; Imbrunito, T.R.; Tomazela, D.M.; De Jesús, M.N.; Calvo Marzal, P.; De Andrade, H.F.; Gustavo Tempone, A. Antimoniais Empregados No Tratamento Da Leishmaniose: Estado Da Arte. Quim. Nova 2003, 26, 550–555. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Tropica 2011, 118, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Teran, R.; Guevara, R.; Mora, J.; Dobronski, L.; Barreiro-Costa, O.; Beske, T.; Pérez-Barrera, J.; Araya-Maturana, R.; Rojas-Silva, P.; Poveda, A.; et al. Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine. Molecules 2019, 24, 2696. [Google Scholar] [CrossRef] [PubMed]
- Gundampati, R.K.; Jagannadham, M. V Molecular Docking Based Inhibition of Trypanothione Reductase Activity by Taxifolin Novel Target for Antileishmanial Activity. J. Appl. Pharm. Sci. 2012, 2, 133–136. [Google Scholar] [CrossRef]
- Schubach, A.O.; Leon, L. Parte VIII-Tratamento e Diagnóstico Das Leishmanioses 23. Modelos de Estudo Para o Desenvolvimento de Drogas Anti-Leishmania; Editora Fiocruz: Rio de Janeiro, Brazil, 2014; pp. 413–428. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, A.; Hussain, I.; Khan, M.A.; Gul, S.; Iqbal, M.; Inayat-Ur-Rahman; Khuda, F. Spectral, XRD, SEM and Biological Properties of New Mononuclear Schiff Base Transition Metal Complexes. Inorg. Chem. Commun. 2013, 35, 104–109. [Google Scholar] [CrossRef]
- Sheikh, S.Y.; Hassan, F.; Shukla, D.; Bala, S.; Faruqui, T.; Akhter, Y.; Khan, A.R.; Nasibullah, M. A Review on Potential Therapeutic Targets for the Treatment of Leishmaniasis. Parasitol. Int. 2024, 100, 102863. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8.14.1–8.14.40. [Google Scholar] [CrossRef] [PubMed]
- Baiocco, P.; Colotti, G.; Franceschini, S.; Ilari, A. Molecular Basis of Antimony Treatment in Leishmaniasis. J. Med. Chem. 2009, 52, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Leimann, F.V.; de Souza, L.B.; de Oliveira, B.P.M.; Rossi, B.F.; da Silva, P.S.; Shiraishi, C.S.H.; Kaplum, V.; Abreu, R.M.; Pereira, C.; Barros, L.; et al. Evaluation of Berberine Nanoparticles as a Strategy to Modulate Acetylcholinesterase Activity. Food Res. Int. 2023, 173, 113295. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q. The Hydrophobic Effects: Our Current Understanding. Molecules 2022, 27, 7009. [Google Scholar] [CrossRef] [PubMed]
- Van der Lubbe, S.C.C.; Fonseca Guerra, C. The Nature of Hydrogen Bonds: A Delineation of the Role of Different Energy Components on Hydrogen Bond Strengths and Lengths. Chem. Asian J. 2019, 14, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully Automated Protein-Ligand Interaction Profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Headen, T.F.; Howard, C.A.; Skipper, N.T.; Wilkinson, M.A.; Bowron, D.T.; Soper, A.K. Structure of π-π Interactions in Aromatic Liquids. J. Am. Chem. Soc. 2010, 132, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, A.S.; Sastry, G.N. Cation-π Interaction: Its Role and Relevance in Chemistry, Biology, and Material Science. Chem. Rev. 2013, 113, 2100–2138. [Google Scholar] [CrossRef] [PubMed]

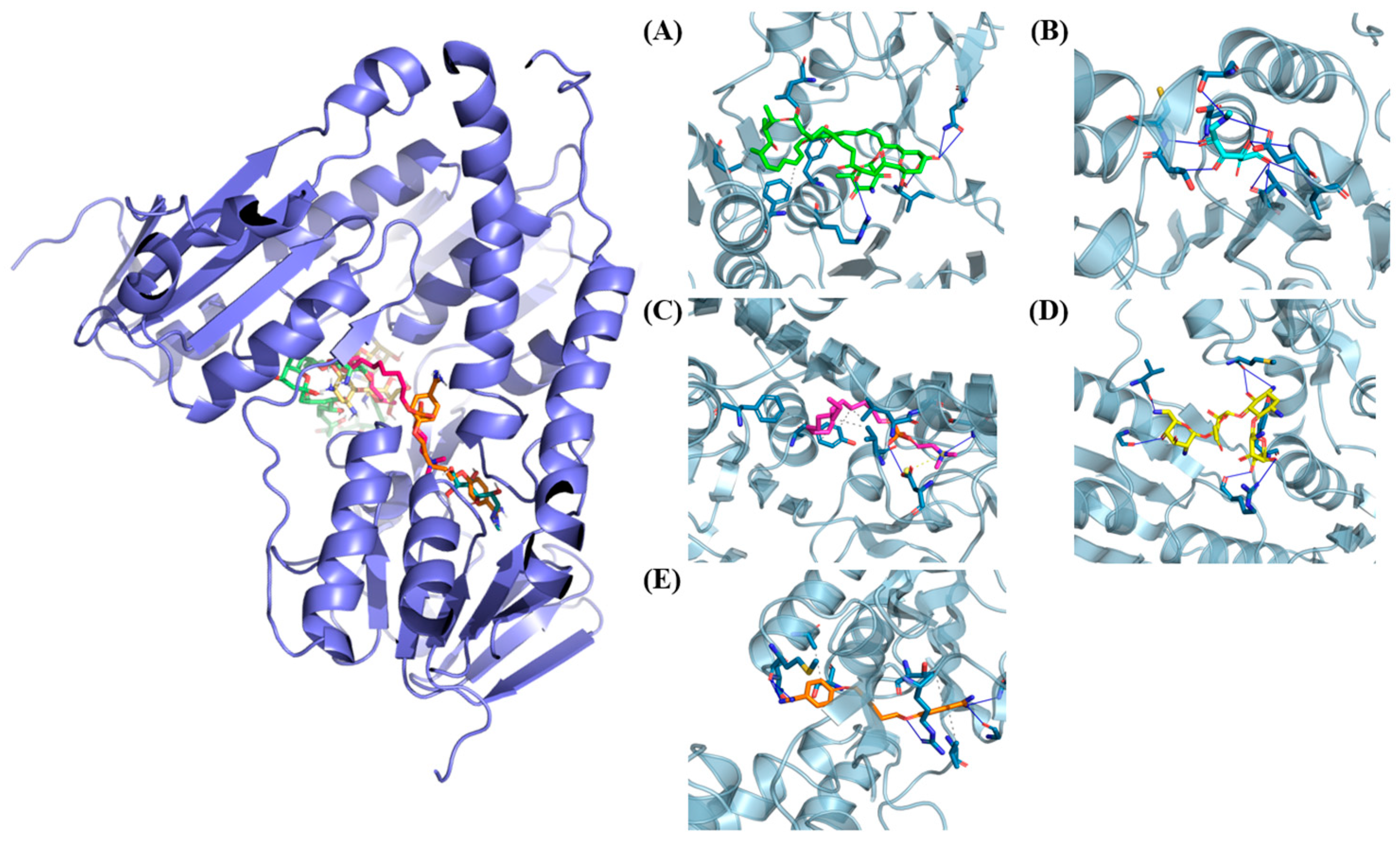
| Code | CID Code | Affinity (kcal/mol) | Interaction Type | Residues |
|---|---|---|---|---|
| Promising Compounds | ||||
| 21 | 265479 | −10.5 | Hydrophobic | Thr51, Lys60, Ile199, Phe203, and Ala338 |
| Hydrogen bond | Ser14, Cys52, Ser178, Tyr198, Asp327, and Thr335 | |||
| 24 | 86573619 | −10.4 | Hydrophobic | Thr51, Lys60, Ile199, Phe203, and Ala338 |
| Hydrogen bond | Ser14, Cys52, Ser178, Tyr198, Asp327, and Thr335 | |||
| 39 | 168349431 | −9.8 | Hydrophobic | Thr51, Lys61, Tyr198, Ile199, Leu334, Ala365, Phe367, and Pro435 |
| Hydrogen bond | Thr355 | |||
| π-Perpendicular | Tyr198 | |||
| π-Cation | Lys60 | |||
| Commercial inhibitors | ||||
| A | Pentamidine | −8.8 | Hydrophobic | Val36, Thr160, and Ala338 |
| Hydrogen | Ser14, Thr51, Cys52, Ala159, Gly161, Asp327, Val328, and Thr335 | |||
| B | Glucantime | −5.9 | Hydrogen | Ser14, Thr51, Cys52, Ala159, Gly161, Asp327, Val328, and Thr335 |
| C | Amphotericin B | −9 | Hydrophobic | Tyr198, Phe230, Leu334, and Thr374 |
| Hydrogen | Arg228, Ile285, and Asn306 | |||
| D | Miltefosine | −5.8 | Hydrophobic | Tyr198, Phe230, Val332 and Leu334 |
| Hydrogen | Ser14, Asp327, and Thr335 | |||
| Salt Bridges | Asp327 | |||
| E | Paromomycin | −6.2 | Hydrogen | Tyr198, Arg228, Met333, Val362, and Gly376 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, D.R.; Shiraishi, C.S.H.; Abreu, R.M.V.; Oliveira, O.V.; dos Santos, J.D. Exploring Schiff Bases as Promising Alternatives to Traditional Drugs in the In Silico Treatment of Anti-Leishmaniasis as Trypanothione Reductase Inhibitors. Proceedings 2024, 102, 55. https://doi.org/10.3390/proceedings2024102055
Peixoto DR, Shiraishi CSH, Abreu RMV, Oliveira OV, dos Santos JD. Exploring Schiff Bases as Promising Alternatives to Traditional Drugs in the In Silico Treatment of Anti-Leishmaniasis as Trypanothione Reductase Inhibitors. Proceedings. 2024; 102(1):55. https://doi.org/10.3390/proceedings2024102055
Chicago/Turabian StylePeixoto, Diego R., Carlos S. H. Shiraishi, Rui M. V. Abreu, Osmair V. Oliveira, and José D. dos Santos. 2024. "Exploring Schiff Bases as Promising Alternatives to Traditional Drugs in the In Silico Treatment of Anti-Leishmaniasis as Trypanothione Reductase Inhibitors" Proceedings 102, no. 1: 55. https://doi.org/10.3390/proceedings2024102055
APA StylePeixoto, D. R., Shiraishi, C. S. H., Abreu, R. M. V., Oliveira, O. V., & dos Santos, J. D. (2024). Exploring Schiff Bases as Promising Alternatives to Traditional Drugs in the In Silico Treatment of Anti-Leishmaniasis as Trypanothione Reductase Inhibitors. Proceedings, 102(1), 55. https://doi.org/10.3390/proceedings2024102055







