Operando Investigations of Rare-Earth Oxycarbonate CO2 Sensors †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis and Sensor Fabrication
2.2. DC Resistance Measurements
2.3. XRD and Operando XRD
3. Results and Discussion
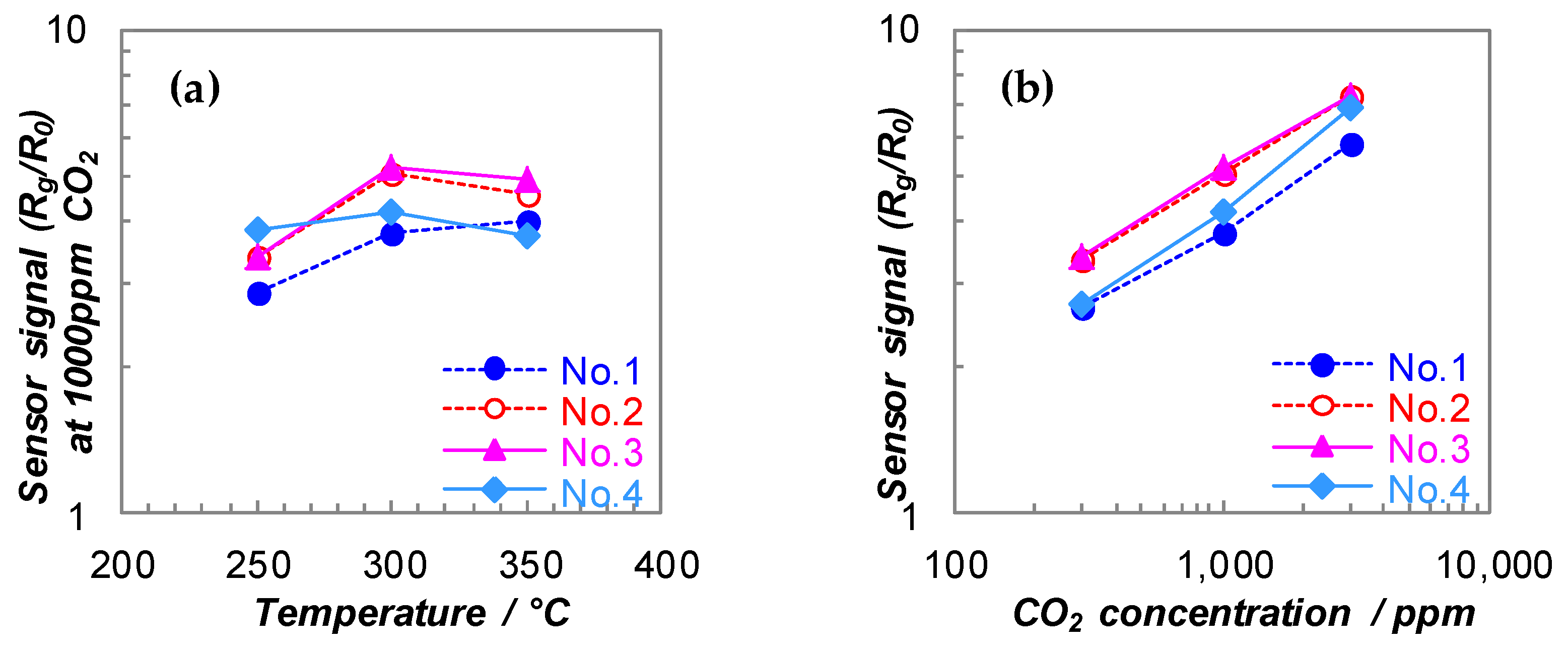
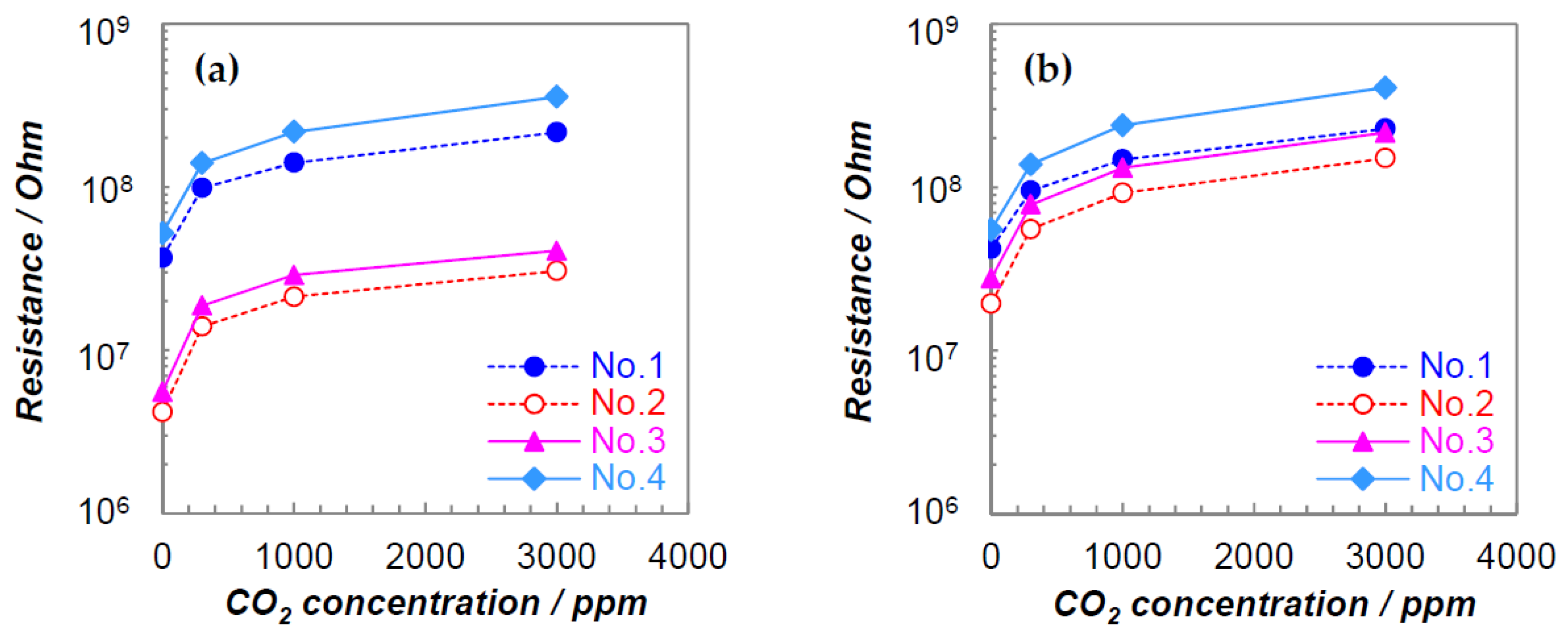
3.1. DC Resistance Measurements
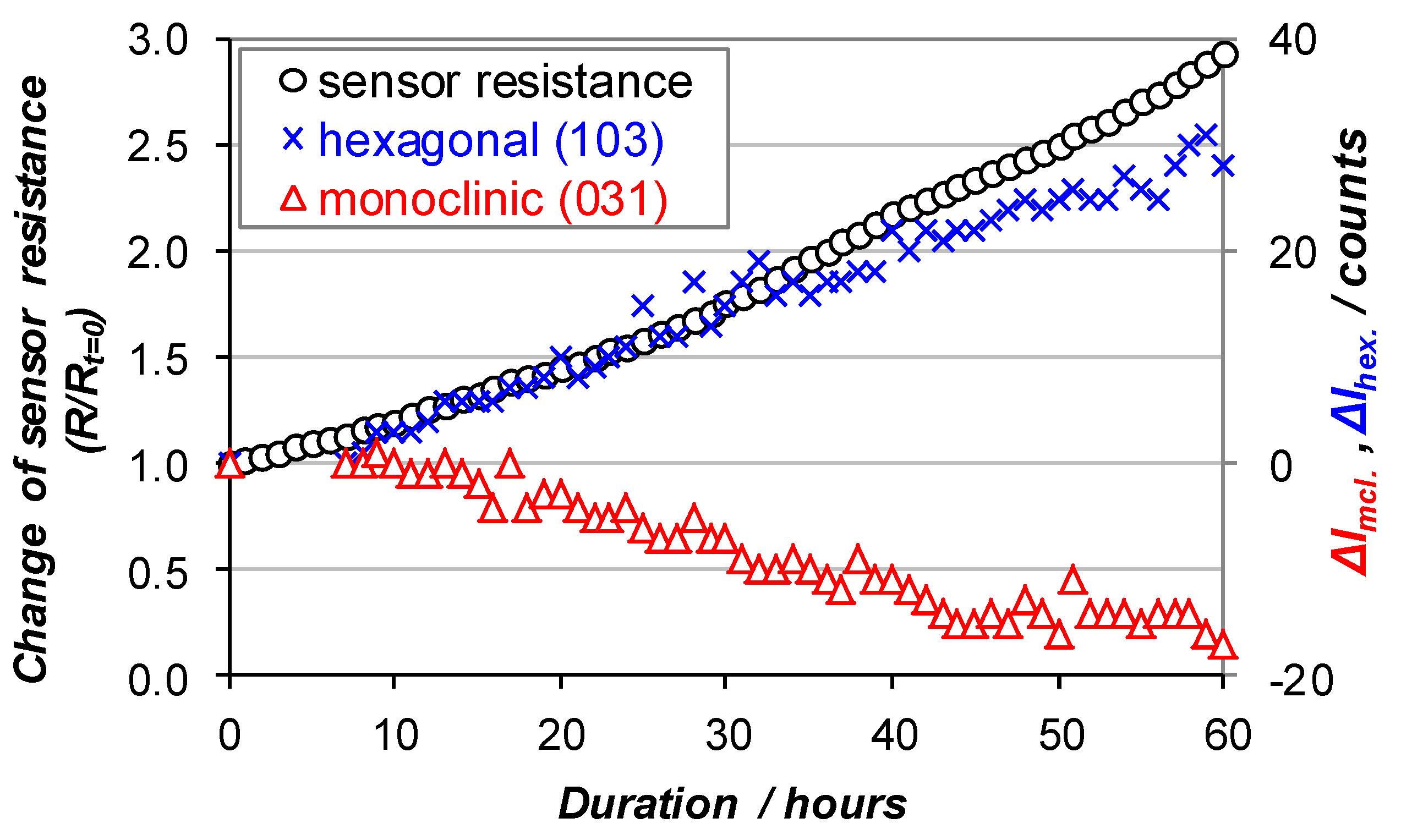
3.2. XRD and Operando XRD
4. Conclusions
Conflicts of Interest
References
- Djerdj, I.; Haensch, A.; Koziej, D.; Pokhrel, S.; Barsan, N.; Weimar, U.; Niederberger, M. Neodymium dioxide carbonate as a sensing layer for chemoresistive CO2 sensing. Chem. Mater. 2009, 21, 5375–5381. [Google Scholar] [CrossRef]
- Haensch, A.; Koziej, D.; Niederberger, M.; Barsan, N.; Weimar, U. Rare earth oxycarbonates as a material class for chemoresistive CO2 gas sensors. Procedia Eng. 2010, 5, 139–142. [Google Scholar] [CrossRef]
- Chen, G.; Han, B.; Deng, S.; Wang, Y.; Wang, Y. Lanthanum dioxide carbonate La2O2CO3 nanorods as a sensing material for chemoresistive CO2 gas sensor. Electrochim. Acta 2014, 127, 355–361. [Google Scholar] [CrossRef]
- Hirsch, O.; Kvashnina, K.O.; Luo, L.; Süess, M.J.; Glatzel, P.; Koziej, D. High-energy resolution X-ray absorption and emission spectroscopy reveals insight into unique selectivity of La-based nanoparticles for CO2. Proc. Natl. Acad. Sci. USA 2015, 112, 15803–15808. [Google Scholar] [CrossRef] [PubMed]
- Kodu, M.; Avarmaa, T.; Mändar, H.; Saar, R.; Jaaniso, R. Structure-Dependent CO2 Gas Sensitivity of La2O2CO3 Thin Films. J. Sens. 2017. [Google Scholar] [CrossRef]
| No. | Starting Material | Heat Treatment Conditions |
|---|---|---|
| 1 | La hydroxide | 450 °C 18 h |
| 2 | La oxalate hydrate | 450 °C 18 h |
| 3 | La oxalate hydrate | 500 °C 18 h |
| 4 | La oxalate hydrate | 500 °C 18 h |
| No. | Before | After |
|---|---|---|
| 1 | La2O2CO3 (h) | La2O2CO3 (h) |
| 2 | La2O2CO3 (m) | La2O2CO3 (m) La2O2CO3 (h) |
| 3 | La2O2CO3 (m) | La2O2CO3 (m) La2O2CO3 (h) |
| 4 | La2O2CO3 (h) | La2O2CO3 (h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Lauxmann, F.; Sackmann, A.; Staerz, A.; Weimar, U.; Berthold, C.; Barsan, N. Operando Investigations of Rare-Earth Oxycarbonate CO2 Sensors. Proceedings 2018, 2, 801. https://doi.org/10.3390/proceedings2130801
Suzuki T, Lauxmann F, Sackmann A, Staerz A, Weimar U, Berthold C, Barsan N. Operando Investigations of Rare-Earth Oxycarbonate CO2 Sensors. Proceedings. 2018; 2(13):801. https://doi.org/10.3390/proceedings2130801
Chicago/Turabian StyleSuzuki, Takuya, Frieder Lauxmann, Andre Sackmann, Anna Staerz, Udo Weimar, Christoph Berthold, and Nicolae Barsan. 2018. "Operando Investigations of Rare-Earth Oxycarbonate CO2 Sensors" Proceedings 2, no. 13: 801. https://doi.org/10.3390/proceedings2130801
APA StyleSuzuki, T., Lauxmann, F., Sackmann, A., Staerz, A., Weimar, U., Berthold, C., & Barsan, N. (2018). Operando Investigations of Rare-Earth Oxycarbonate CO2 Sensors. Proceedings, 2(13), 801. https://doi.org/10.3390/proceedings2130801




