Apta- and Immuno-Sensors Performance Optimization: A Comparative Study of Surface Functionalization Techniques †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Silanization and (Bio)-Chemical Modifications
2.1.1. Aptamer-Based Sensors
2.1.2. Antibody-Based Sensors
2.2. Aptamer and Antibody Immobilization
2.3. Visualization of the Immobilized and Captured Biomolecules
3. Results and Discussion
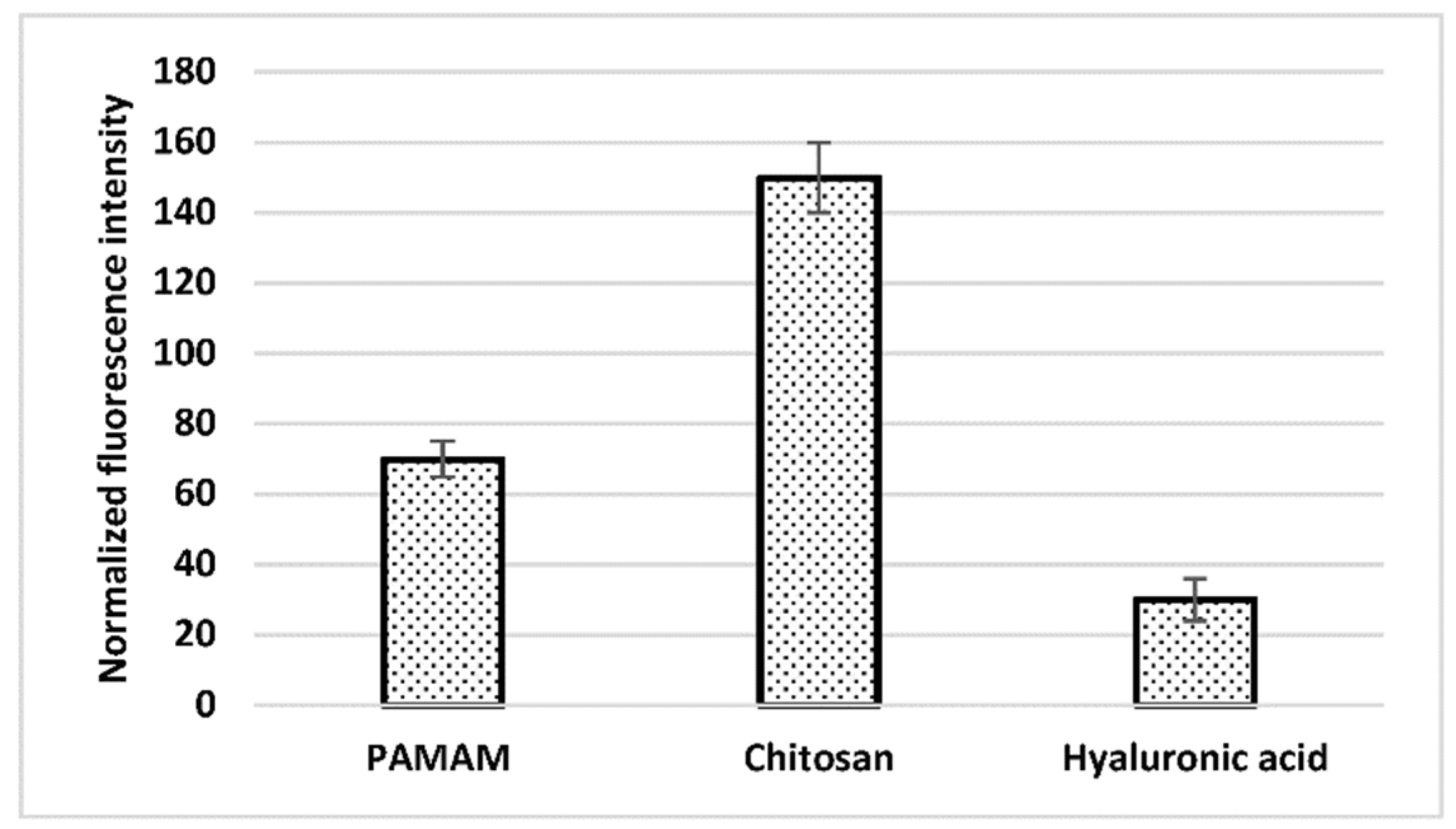
3.1. Apta-Sensors
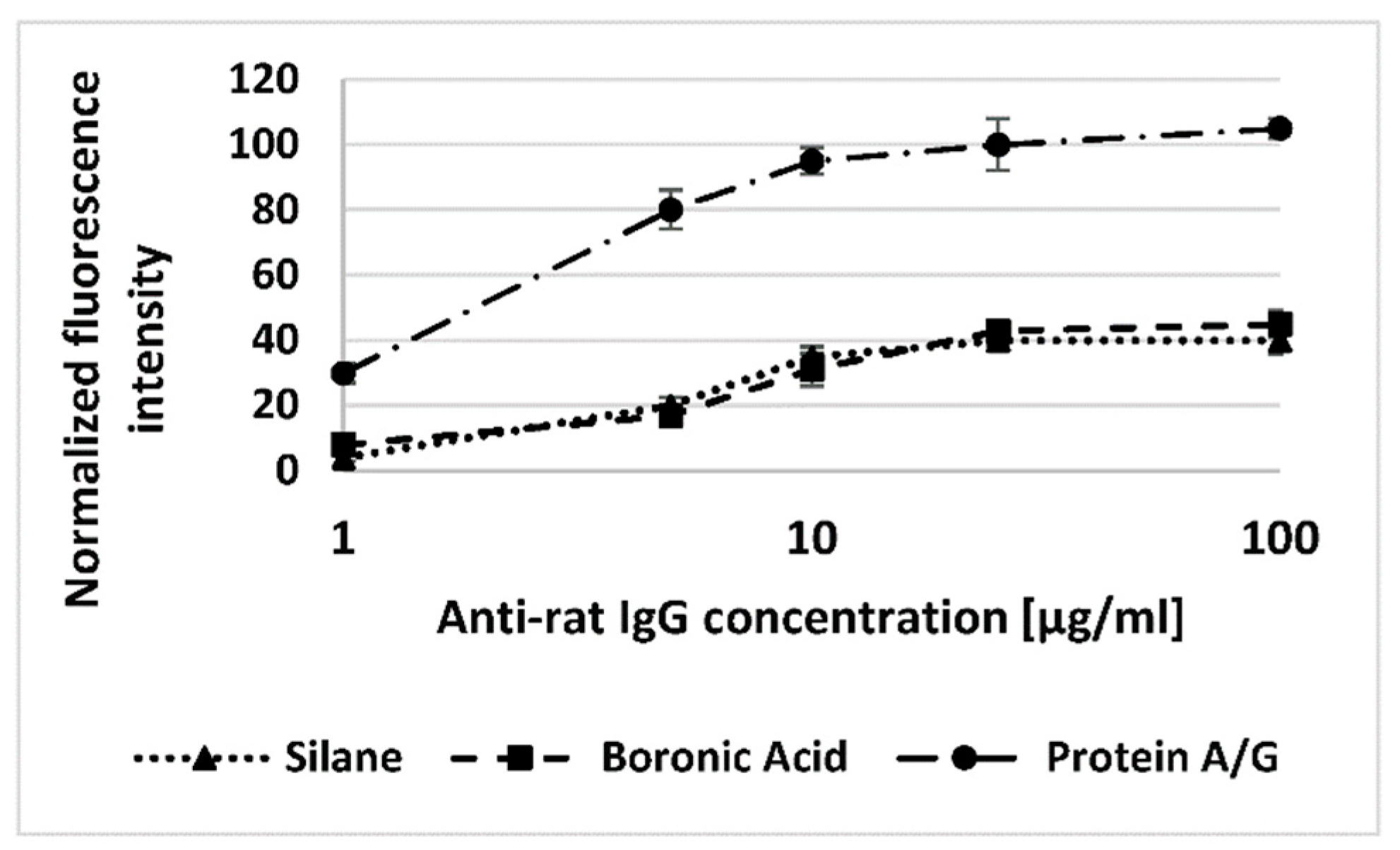
3.2. Immunosensors
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Tiwari, A.; Choi, J.W.; Pandey, A.C. Smart nanomaterials for biosensors, biochips and molecular bioelectronics. In Smart Nanomaterials for Sensor Applications, 2nd ed.; Li, S., Ge, Y., Li, H., Eds.; Bentham Science Publishers: Beijing, China, 2012; pp. 3–41. [Google Scholar]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody orientation on biosensor surfaces: A minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Aguado, J.A.; Penner, G. Determination of Ochratoxin A with a DNA Aptamer. J. Agric. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef] [PubMed]
- Tsekenis, G.; Chatzipetrou, M.; Tanner, J.; Chatzandroulis, S.; Thanos, D.; Tsoukalas, D.; Zergioti, I. Surface functionalization studies and direct laser printing of oligonucleotides toward the fabrication of a micromembrane DNA capacitive biosensor. Sens. Actuators B Chem. 2012, 175, 123–131. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsekenis, G.; Vanikioti, V.; Kordatos, K.; Zergioti, I. Apta- and Immuno-Sensors Performance Optimization: A Comparative Study of Surface Functionalization Techniques. Proceedings 2018, 2, 847. https://doi.org/10.3390/proceedings2130847
Tsekenis G, Vanikioti V, Kordatos K, Zergioti I. Apta- and Immuno-Sensors Performance Optimization: A Comparative Study of Surface Functionalization Techniques. Proceedings. 2018; 2(13):847. https://doi.org/10.3390/proceedings2130847
Chicago/Turabian StyleTsekenis, George, Valentina Vanikioti, Konstantinos Kordatos, and Ioanna Zergioti. 2018. "Apta- and Immuno-Sensors Performance Optimization: A Comparative Study of Surface Functionalization Techniques" Proceedings 2, no. 13: 847. https://doi.org/10.3390/proceedings2130847
APA StyleTsekenis, G., Vanikioti, V., Kordatos, K., & Zergioti, I. (2018). Apta- and Immuno-Sensors Performance Optimization: A Comparative Study of Surface Functionalization Techniques. Proceedings, 2(13), 847. https://doi.org/10.3390/proceedings2130847







