Investigation of Electrochemical Processes in CO2 Sensitive Electrodes †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
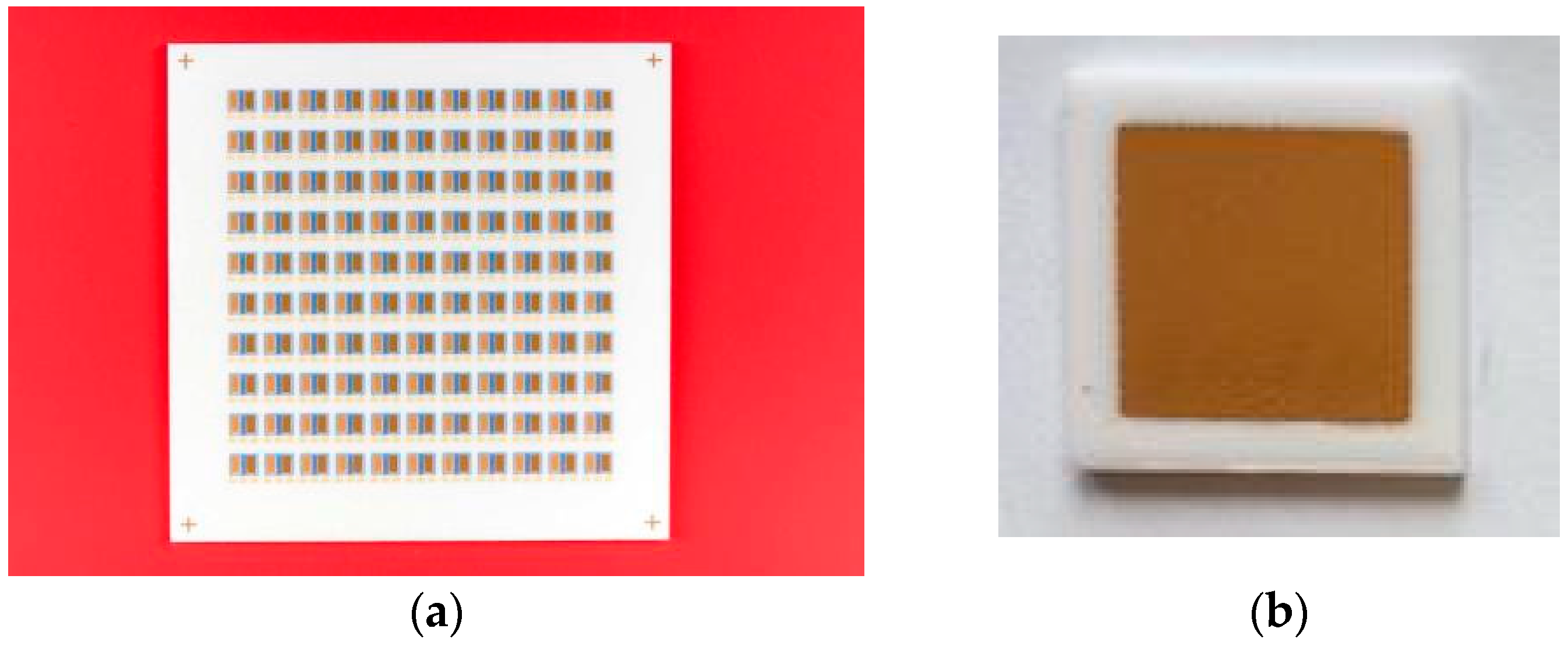
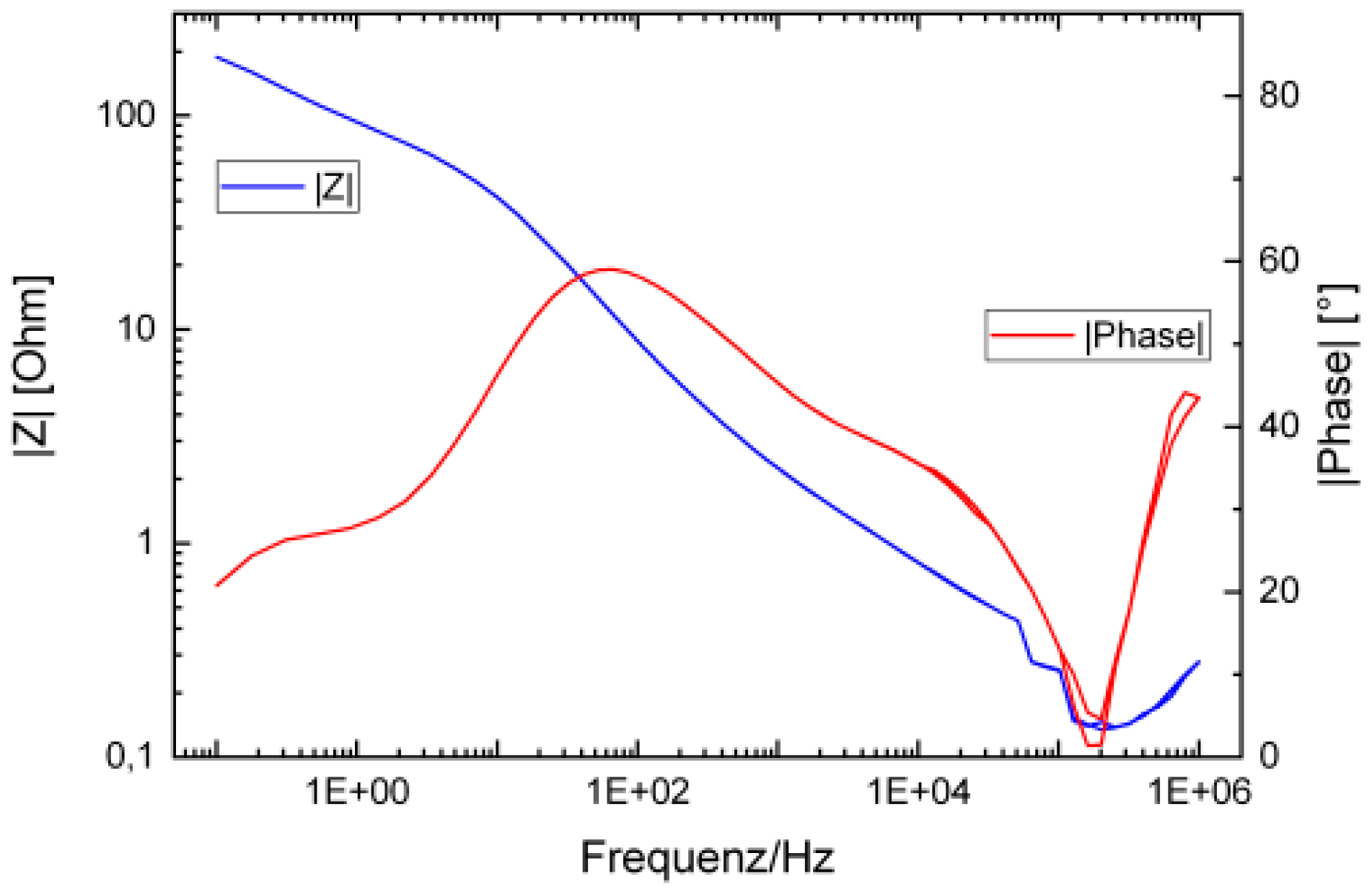
3.1. Sensor Characterization
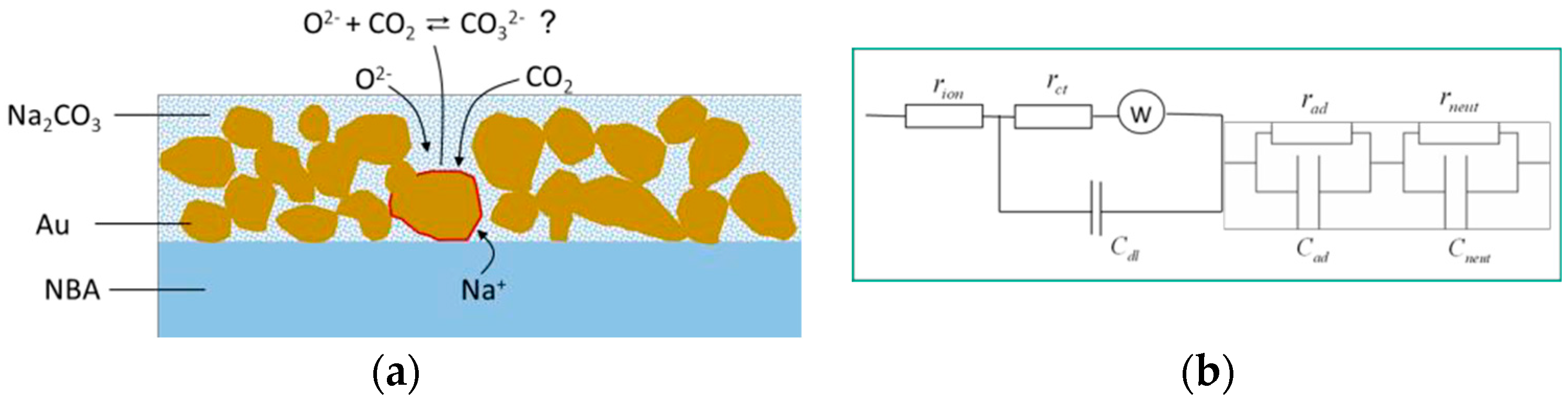
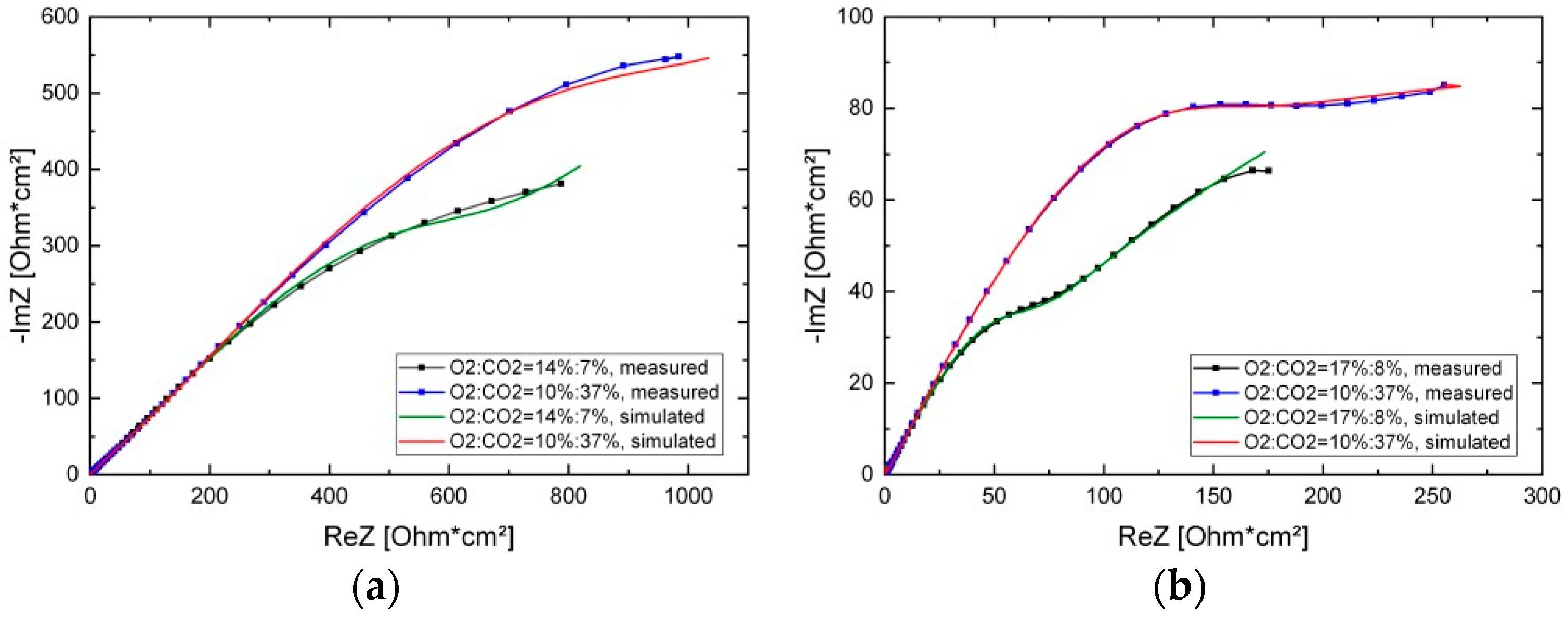
3.2. EIS Measurements
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Meléndez-Ceballos, A.; Albin, V. Kinetic approach on the effect of Cs addition on oxygen reduction for MCFC application. Electrochim. Acta 2015, 184, 295–300. [Google Scholar] [CrossRef]
- Bednarz, M. Mechanistische Untersuchung und Modellierung der Kathodenreaktion in Karbonat-brennstoffzellen (MCFC). Ph.D. Thesis, IPCH Universität Hamburg, Hamburg, Germany, 2002. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, S.; Kusnezoff, M.; Mosch, S.; Baumgärtner, C. Investigation of Electrochemical Processes in CO2 Sensitive Electrodes. Proceedings 2018, 2, 903. https://doi.org/10.3390/proceedings2130903
Dietrich S, Kusnezoff M, Mosch S, Baumgärtner C. Investigation of Electrochemical Processes in CO2 Sensitive Electrodes. Proceedings. 2018; 2(13):903. https://doi.org/10.3390/proceedings2130903
Chicago/Turabian StyleDietrich, Stefan, Mihails Kusnezoff, Sindy Mosch, and Christoph Baumgärtner. 2018. "Investigation of Electrochemical Processes in CO2 Sensitive Electrodes" Proceedings 2, no. 13: 903. https://doi.org/10.3390/proceedings2130903
APA StyleDietrich, S., Kusnezoff, M., Mosch, S., & Baumgärtner, C. (2018). Investigation of Electrochemical Processes in CO2 Sensitive Electrodes. Proceedings, 2(13), 903. https://doi.org/10.3390/proceedings2130903



