Iron Oxide Nanoparticle Decorated Graphene for Ultra-Sensitive Detection of Volatile Organic Compounds †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensing Layer Preparation
2.2. Characterization Techniques
2.3. Sensor Device Fabrication
3. Results and Discussion
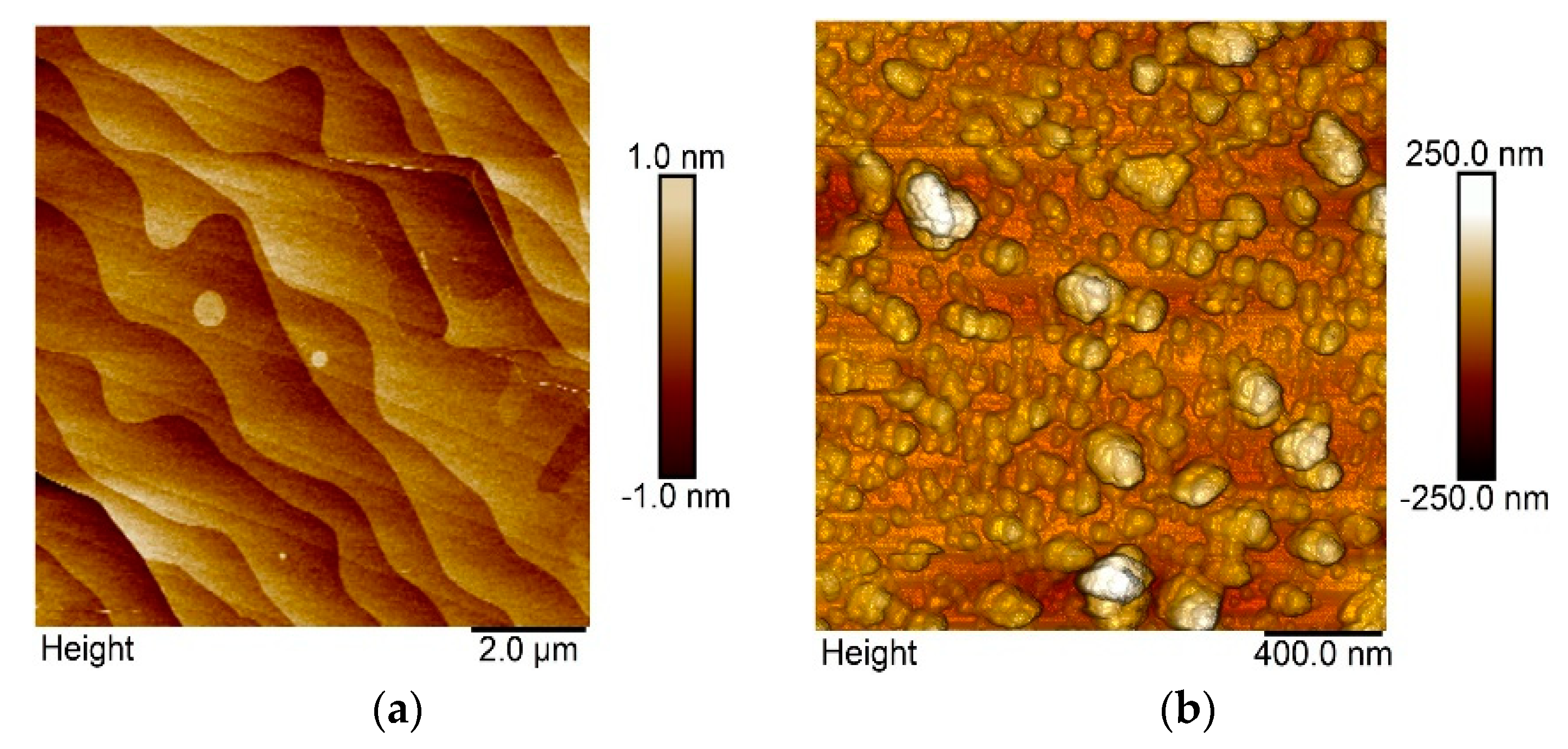
3.1. Morphological and Structural Characterization
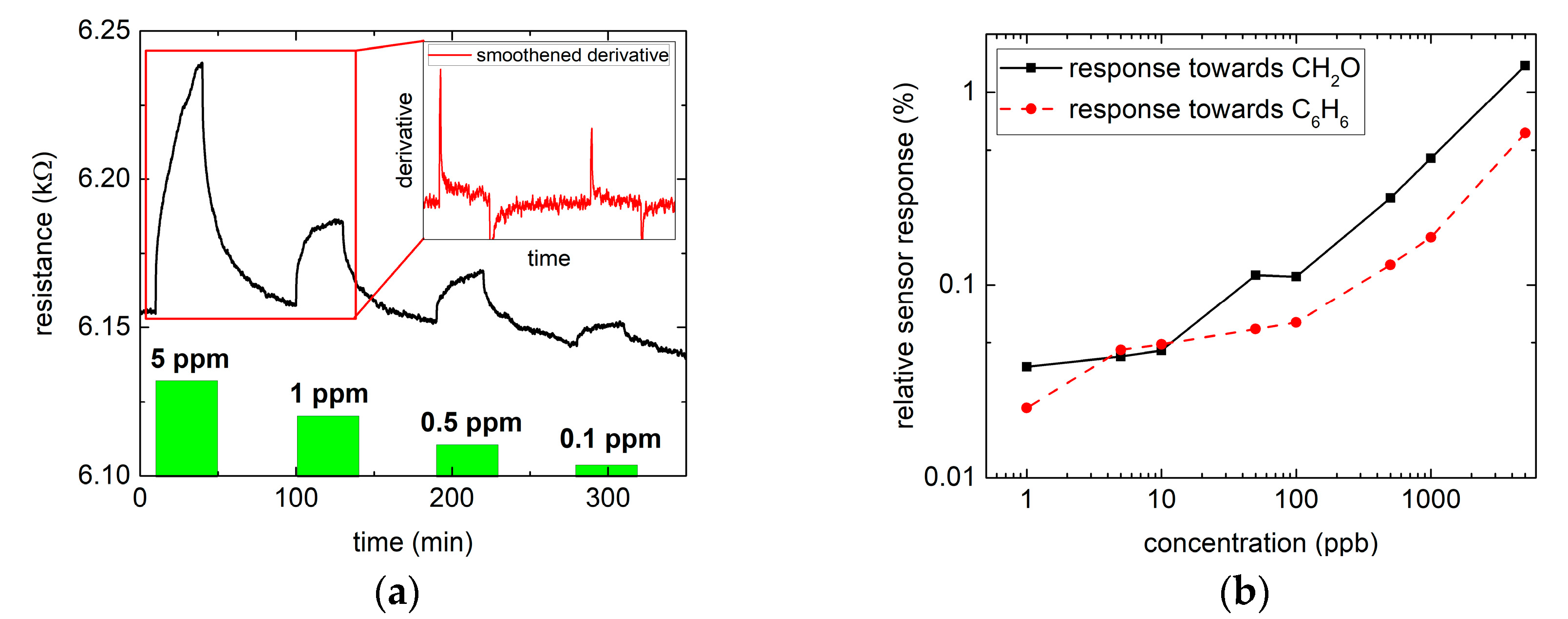
3.2. Gas Measurements
 , where R is the saturate resistance signal and R0 corresponds to the baseline resistance before the gas exposure. The relative response for different concentrations of benzene and formaldehyde is shown in Figure 2b. A distinct response for both gases over the whole range can clearly be observed. Moreover, the safety limit for formaldehyde (~80 ppb) can easily be reached and also benzene (no safety level reported) can be quantitatively measured down to a single ppb. The relative responses towards 1 ppb formaldehyde and benzene are about 0.04% and 0.02%, respectively.
, where R is the saturate resistance signal and R0 corresponds to the baseline resistance before the gas exposure. The relative response for different concentrations of benzene and formaldehyde is shown in Figure 2b. A distinct response for both gases over the whole range can clearly be observed. Moreover, the safety limit for formaldehyde (~80 ppb) can easily be reached and also benzene (no safety level reported) can be quantitatively measured down to a single ppb. The relative responses towards 1 ppb formaldehyde and benzene are about 0.04% and 0.02%, respectively.4. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Atlas on Children’s Health and the Environment; World Health Organisation: Geneva, Switzerland, 2017; ISBN 9789241511773. [Google Scholar]
- World Health Organisation. Economic Cost of the Health Impact of Air Pollution in Europe: Clean Air, Health and Wealth; World Health Organisation: Copenhagen, Danmark, 2015. [Google Scholar]
- Szulczyński, B.; Gębicki, J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Eriksson, J.; Puglisi, D.; Strandqvist, C.; Gunnarsson, R.; Ekeroth, S. Modified Epitaxial Graphene on SiC for Extremely Sensitive and Selective Gas Sensors. Mater. Sci. Forum 2016, 858, 1145–1148. [Google Scholar] [CrossRef]
- Rodner, M.; Bahonjic, J.; Mathisen, M.; Gunnarsson, R.; Ekeroth, S.; Helmersson, U.; Ivanov, I.G.; Yakimova, R.; Eriksson, J. Performance tuning of gas sensors based on epitaxial graphene on silicon carbide. Mater. Des. 2018, 153, 153–158. [Google Scholar] [CrossRef]
- Yakimova, R.; Virojanadara, C.; Gogova, D.; Syväjärvi, M.; Siche, D.; Larsson, K.; Johansson, L. Analysis of the Formation Conditions for Large Area Epitaxial Graphene on SiC Substrates. Mater. Sci. Forum 2010, 645–648, 565–568. [Google Scholar] [CrossRef]
- Pilch, I.; Söderström, D.; Hasan, M.I.; Helmersson, U.; Brenning, N. Fast growth of nanoparticles in a hollow cathode plasma through orbit motion limited ion collection. Appl. Phys. Lett. 2013, 103, 193108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodner, M.; Puglisi, D.; Ekeroth, S.; Helmersson, U.; Ivanov, I.G.; Yakimova, R.; Uvdal, K.; Schütze, A.; Eriksson, J. Iron Oxide Nanoparticle Decorated Graphene for Ultra-Sensitive Detection of Volatile Organic Compounds. Proceedings 2018, 2, 985. https://doi.org/10.3390/proceedings2130985
Rodner M, Puglisi D, Ekeroth S, Helmersson U, Ivanov IG, Yakimova R, Uvdal K, Schütze A, Eriksson J. Iron Oxide Nanoparticle Decorated Graphene for Ultra-Sensitive Detection of Volatile Organic Compounds. Proceedings. 2018; 2(13):985. https://doi.org/10.3390/proceedings2130985
Chicago/Turabian StyleRodner, Marius, Donatella Puglisi, Sebastian Ekeroth, Ulf Helmersson, Ivan G. Ivanov, Rositsa Yakimova, Kajsa Uvdal, Andreas Schütze, and Jens Eriksson. 2018. "Iron Oxide Nanoparticle Decorated Graphene for Ultra-Sensitive Detection of Volatile Organic Compounds" Proceedings 2, no. 13: 985. https://doi.org/10.3390/proceedings2130985
APA StyleRodner, M., Puglisi, D., Ekeroth, S., Helmersson, U., Ivanov, I. G., Yakimova, R., Uvdal, K., Schütze, A., & Eriksson, J. (2018). Iron Oxide Nanoparticle Decorated Graphene for Ultra-Sensitive Detection of Volatile Organic Compounds. Proceedings, 2(13), 985. https://doi.org/10.3390/proceedings2130985





