Effect of Nanostructured Octahedral SnO2 Added with a Binary Mixture P-Type and N-Type Metal Oxide on CO Detection †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Jońca, J.; Ryzhikov, A.; Kahn, M.L.; Fajerwerg, K.; Chapelle, A.; Menini, P.; Fau, P. SnO2 “Russian Doll” Octahedra Prepared by Metalorganic Synthesis: A New Structure for Sub-ppm CO Detection. Chem. A Eur. J. 2016, 22, 10127–10135. [Google Scholar] [CrossRef]
- Jońca, J.; Ryzhikov, A.; Palussière, S.; Esvan, J.; Fajerwerg, K.; Menini, P.; Kahn, M.L.; Fau, P. Organometallic Synthesis of CuO Nanoparticles: Application in Low-Temperature CO Detection. Chem. Phys. Chem. 2017, 18, 2658–2665. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Jońca, J.; Kahn, M.; Fajerwerg, K.; Chaudret, B.; Chapelle, A.; Ménini, P.; Shim, C.H.; Gaudon, A.; Fau, P. Organometallic synthesis of ZnO nanoparticles for gas sensing: towards selectivity through nanoparticles morphology. J. Nanopart. Res. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Dufour, N.; Chapelle, A.; Mesnilgrente, F.; Conedera, V.; Menini, P. Technological improvements of a metal oxide gas multi-sensor based on a micro-hotplate structure and inkjet deposition for an automotive air quality sensor application. In Proceedings of the 25th Micromechanics and Microsystems Europe Workshop, MME 2014, Istanbul, Turkey, August 31–September 3, 2014. [Google Scholar]
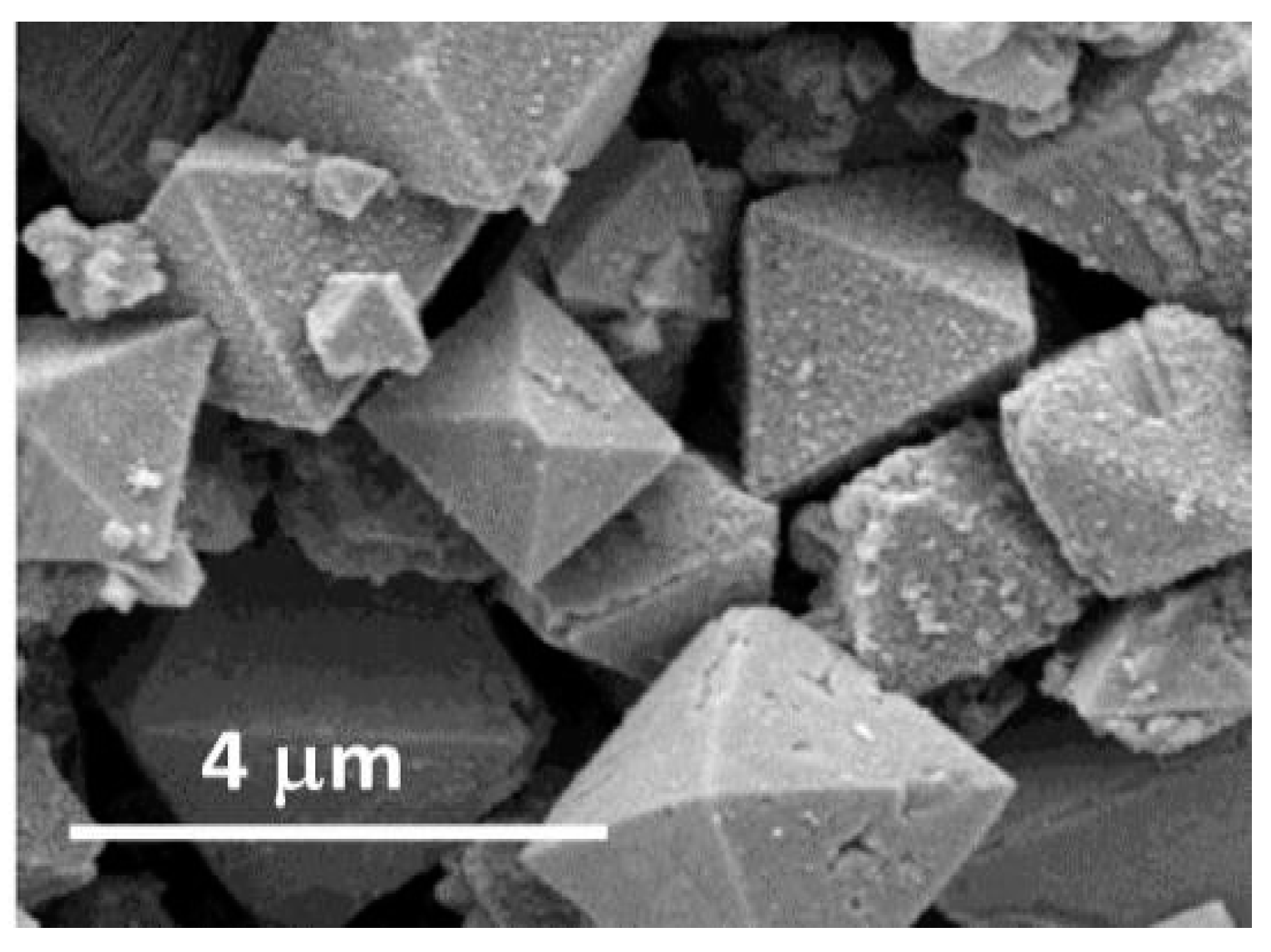



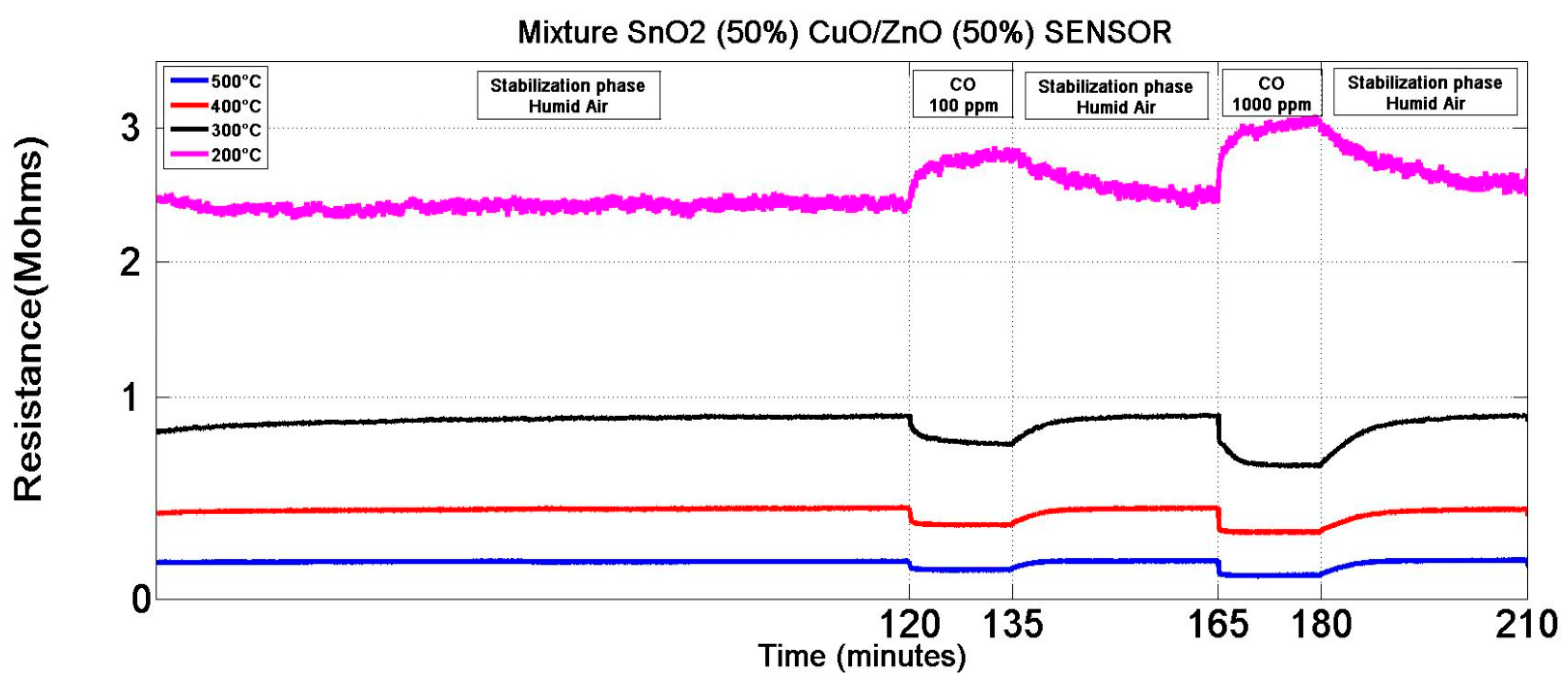
| Sensor | Type | MOX Blend (% mass) |
|---|---|---|
| Sensor 1 | Binary mixture | CuO75%/ZnO25% |
| Sensor 2 | Ternary mixture | SnO2 (25%) CuO75%/ZnO25% (75%) |
| Sensor 3 | Ternary mixture | SnO2 (50%) CuO75%/ZnO25% (50%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sendi, A.; Menini, P.; Kahn, M.L.; Fajerwerg, K.; Fau, P. Effect of Nanostructured Octahedral SnO2 Added with a Binary Mixture P-Type and N-Type Metal Oxide on CO Detection. Proceedings 2018, 2, 986. https://doi.org/10.3390/proceedings2130986
Sendi A, Menini P, Kahn ML, Fajerwerg K, Fau P. Effect of Nanostructured Octahedral SnO2 Added with a Binary Mixture P-Type and N-Type Metal Oxide on CO Detection. Proceedings. 2018; 2(13):986. https://doi.org/10.3390/proceedings2130986
Chicago/Turabian StyleSendi, Aymen, Philippe Menini, Myrtil L. Kahn, Katia Fajerwerg, and Pierre Fau. 2018. "Effect of Nanostructured Octahedral SnO2 Added with a Binary Mixture P-Type and N-Type Metal Oxide on CO Detection" Proceedings 2, no. 13: 986. https://doi.org/10.3390/proceedings2130986
APA StyleSendi, A., Menini, P., Kahn, M. L., Fajerwerg, K., & Fau, P. (2018). Effect of Nanostructured Octahedral SnO2 Added with a Binary Mixture P-Type and N-Type Metal Oxide on CO Detection. Proceedings, 2(13), 986. https://doi.org/10.3390/proceedings2130986





