1,3-Di(hetero)aryl-7-substituted Pyrenes—An Undiscovered Area of Important Pyrene Derivatives †
Abstract
:1. Introduction
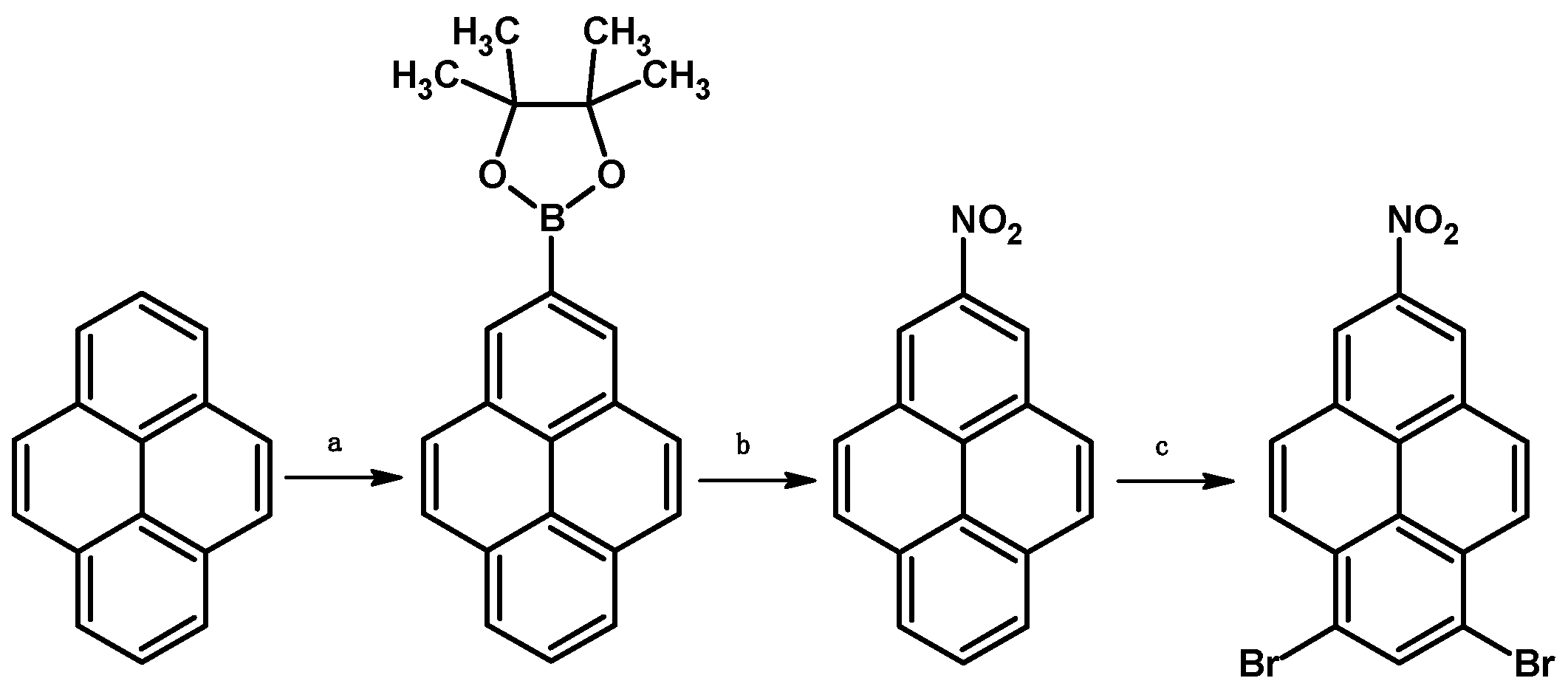
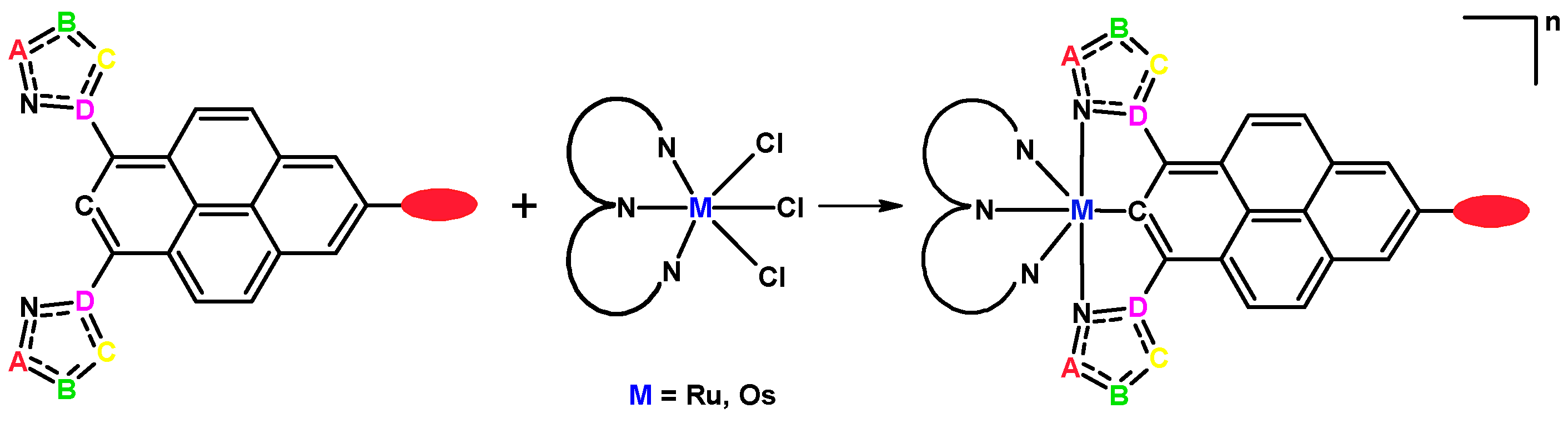
2. Results and Discussion
3. Conclusions
4. Methods
Acknowledgments
References
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hu, J.-Y.; Redshaw, C.; Yamato, T. Functionalization of Pyrene To Prepare Luminescent Materials-Typical Examples of Synthetic Methodology. Chem.-Eur. J. 2016, 22, 11898–11916. [Google Scholar] [CrossRef] [PubMed]
- Casas-Solvas, J.M.; Howgego, J.D.; Davis, A.P. Synthesis of substituted pyrenes by indirect methods. Org. Biomol. Chem. 2014, 12, 212–232. [Google Scholar] [CrossRef] [PubMed]
- Zych, D. Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review. Molecules 2019, 24, 2551. [Google Scholar] [CrossRef] [PubMed]
- Zych, D.; Kurpanik, A.; Slodek, A.; Maroń, A.; Pająk, M.; Szafraniec-Gorol, G.; Matussek, M.; Krompiec, S.; Schab-Balcerzak, E.; Kotowicz, S.; et al. NCN-Coordinating Ligands based on Pyrene Structure with Potential Application in Organic Electronics. Chem.-Eur. J. 2017, 23, 15746–15758. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Kimura, M. Low band gap disk-shaped donors for solution-processed organic solar cells. RSC Adv. 2014, 4, 64589–64595. [Google Scholar] [CrossRef]
- Lorbach, D.; Keerthi, A.; Figueira-Duarte, T.M.; Baumgarten, M.; Wagner, M.; Müllen, K. Cyclization of Pyrene Oligomers: Cyclohexa-1,3-pyrenylene. Angew. Chemie Int. Ed. 2016, 55, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hu, J.-Y.; Tomiyasu, H.; Tao, Z.; Redshaw, C.; Elsegood, M.R.J.; Horsburgh, L.; Teat, S.J.; Wei, X.-F.; Yamato, T. Iron( iii ) bromide catalyzed bromination of 2-tert-butylpyrene and corresponding position-dependent aryl-functionalized pyrene derivatives. RSC Adv. 2015, 5, 8835–8848. [Google Scholar] [CrossRef]
- Gong, X.; Pan, Y.; Xie, X.; Tong, T.; Chen, R.; Gao, D. Synthesis, characterization and electroluminescence of two highly-twisted non-doped blue light-emitting materials. Opt. Mater. (Amst.) 2018, 78, 94–101. [Google Scholar] [CrossRef]
- Li, X.; Deng, X.; Coyne, A.G.; Srinivasan, R. meta -Nitration of Arenes Bearing ortho / para Directing Group(s) Using C−H Borylation. Chem.-Eur. J. 2019, 25, 8018–8023. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Takeda, T.; Kuratsu, M.; Kozaki, M.; Sato, K.; Shiomi, D.; Takui, T.; Okada, K. Pyrene-Dihydrophenazine Bis(Radical Cation) in a Singlet Ground State. Org. Lett. 2009, 11, 2816–2818. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.G.; Liu, Z.; Mkhalid, I.A.I.; Thibault, M.; Schwarz, N.; Alcaraz, G.; Steffen, A.; Collings, J.C.; Batsanov, A.S.; Howard, J.A.K.; et al. Synthesis of 2- and 2,7-Functionalized Pyrene Derivatives: An Application of Selective C-H Borylation. Chem.-Eur. J. 2012, 18, 5022–5035. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lorbach, A.; Edkins, R.M.; Marder, T.B. Synthesis and Photophysics of a 2,7-Disubstituted Donor–Acceptor Pyrene Derivative: An Example of the Application of Sequential Ir-Catalyzed C–H Borylation and Substitution Chemistry. J. Org. Chem. 2015, 80, 5658–5665. [Google Scholar] [CrossRef] [PubMed]
- Zych, D.; Slodek, A.; Golba, S.; Krompiec, S. Cyclometalated Ruthenium, Osmium, and Iridium Complexes Bridged by an NCN-Pyrene-NCN Derivative—Synthesis and Comparison of Optical, Thermal, and Electrochemical Properties. Eur. J. Inorg. Chem. 2018, 2018, 1581–1588. [Google Scholar] [CrossRef]
- Zych, D.; Slodek, A.; Matuszczyk, D.; Golba, S. Comprehensive Study of Mononuclear Osmium Complexes with Various Pyrene Ligands. Eur. J. Inorg. Chem. 2018, 2018, 5117–5128. [Google Scholar] [CrossRef]
- Frisch; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revis. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]



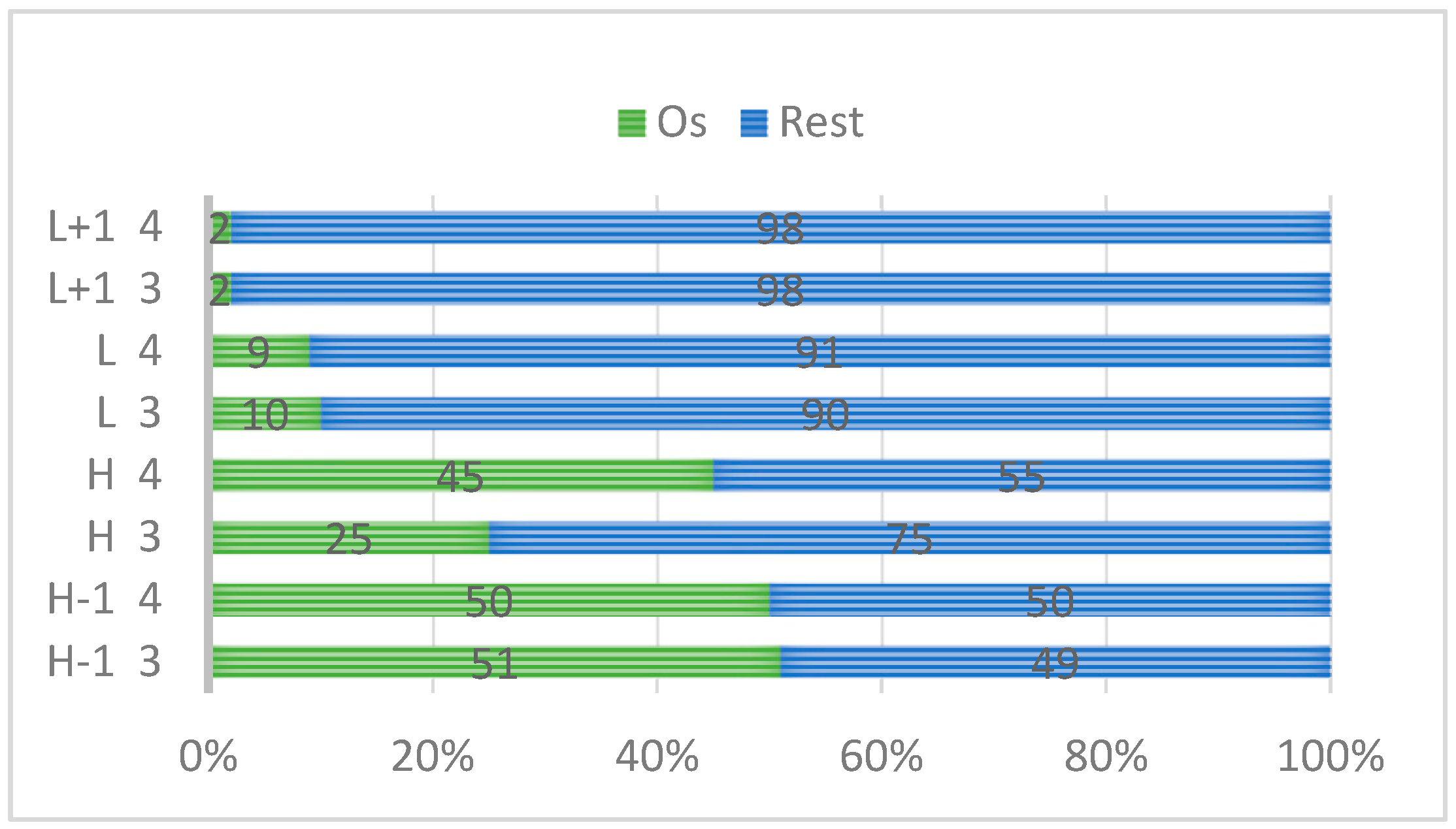
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| H-1 | 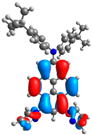 |  |  | 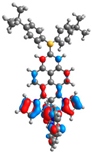 |
| −5.50 | −6.40 | −5.08 | −5.06 | |
| H | 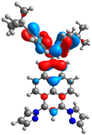 | 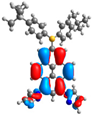 | 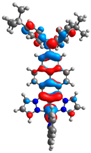 | 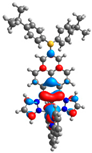 |
| −5.05 | −5.49 | −4.78 | −4.98 | |
| L |  | 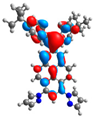 | 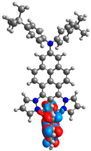 | 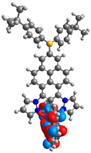 |
| −1.92 | −1.92 | −2.22 | −2.24 | |
| L+1 | 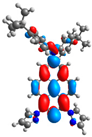 | 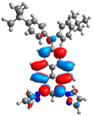 | 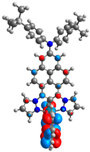 |  |
| −1.04 | −1.91 | −2.14 | −2.15 | |
| ΔE | 3.13 | 3.57 | 2.56 | 2.74 |
| 3 | 4 |
|---|---|
 | 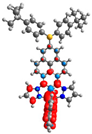 |
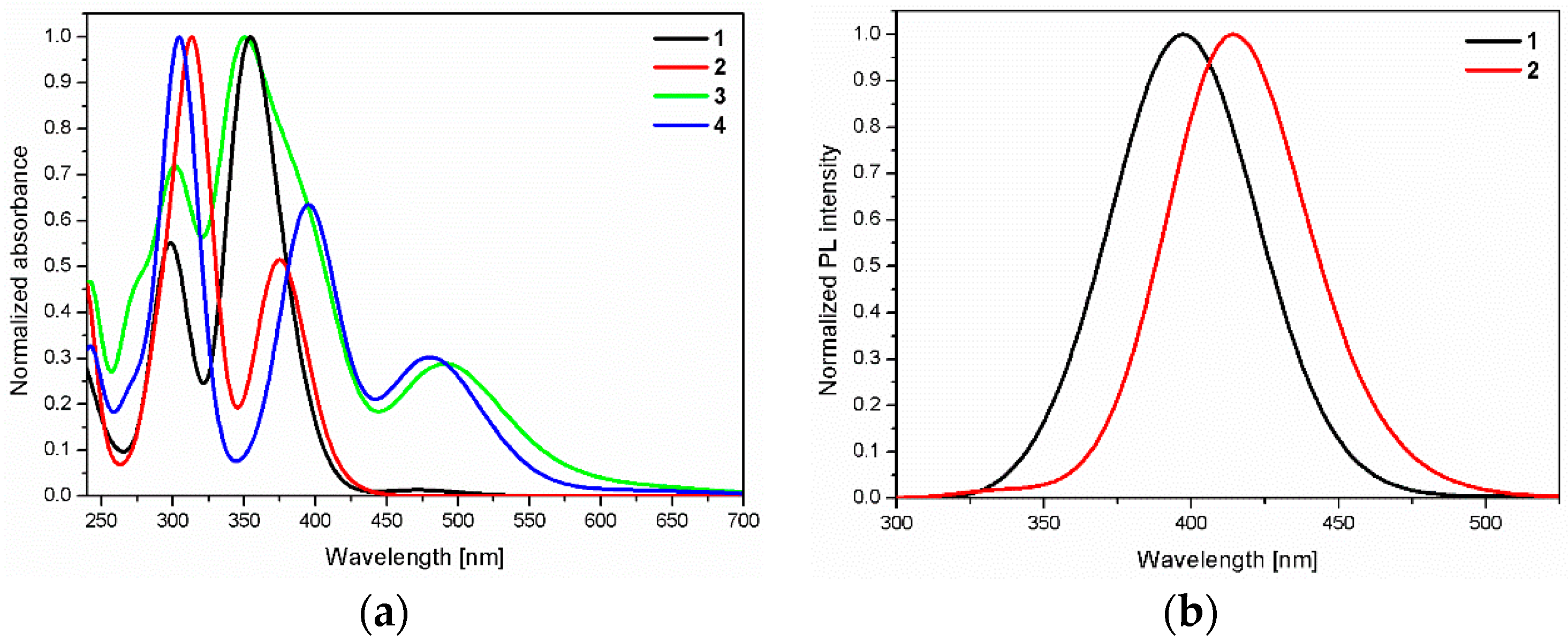
| λmax [nm] | PL λem [nm] | ||
|---|---|---|---|
| 1 | 299, 355 | 397 | 404.22 (0.8173) H-1->LUMO (95%) 386.47 (0.5091) HOMO->L+1 (95%) |
| 2 | 313, 375 | 415 | 414.57 (0.9983) HOMO->L+1 (97%) |
| 3 | 273sh, 302, 350, 390sh, 491 | - | - |
| 4 | 305, 395, 479 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, D. 1,3-Di(hetero)aryl-7-substituted Pyrenes—An Undiscovered Area of Important Pyrene Derivatives. Proceedings 2019, 41, 28. https://doi.org/10.3390/ecsoc-23-06470
Zych D. 1,3-Di(hetero)aryl-7-substituted Pyrenes—An Undiscovered Area of Important Pyrene Derivatives. Proceedings. 2019; 41(1):28. https://doi.org/10.3390/ecsoc-23-06470
Chicago/Turabian StyleZych, Dawid. 2019. "1,3-Di(hetero)aryl-7-substituted Pyrenes—An Undiscovered Area of Important Pyrene Derivatives" Proceedings 41, no. 1: 28. https://doi.org/10.3390/ecsoc-23-06470
APA StyleZych, D. (2019). 1,3-Di(hetero)aryl-7-substituted Pyrenes—An Undiscovered Area of Important Pyrene Derivatives. Proceedings, 41(1), 28. https://doi.org/10.3390/ecsoc-23-06470





