New Quaternary Chalcogenides Tl2MIIMIV3Se8 and Tl2MIIMIVX4 †
Abstract
:1. Introduction
2. Materials and Methods
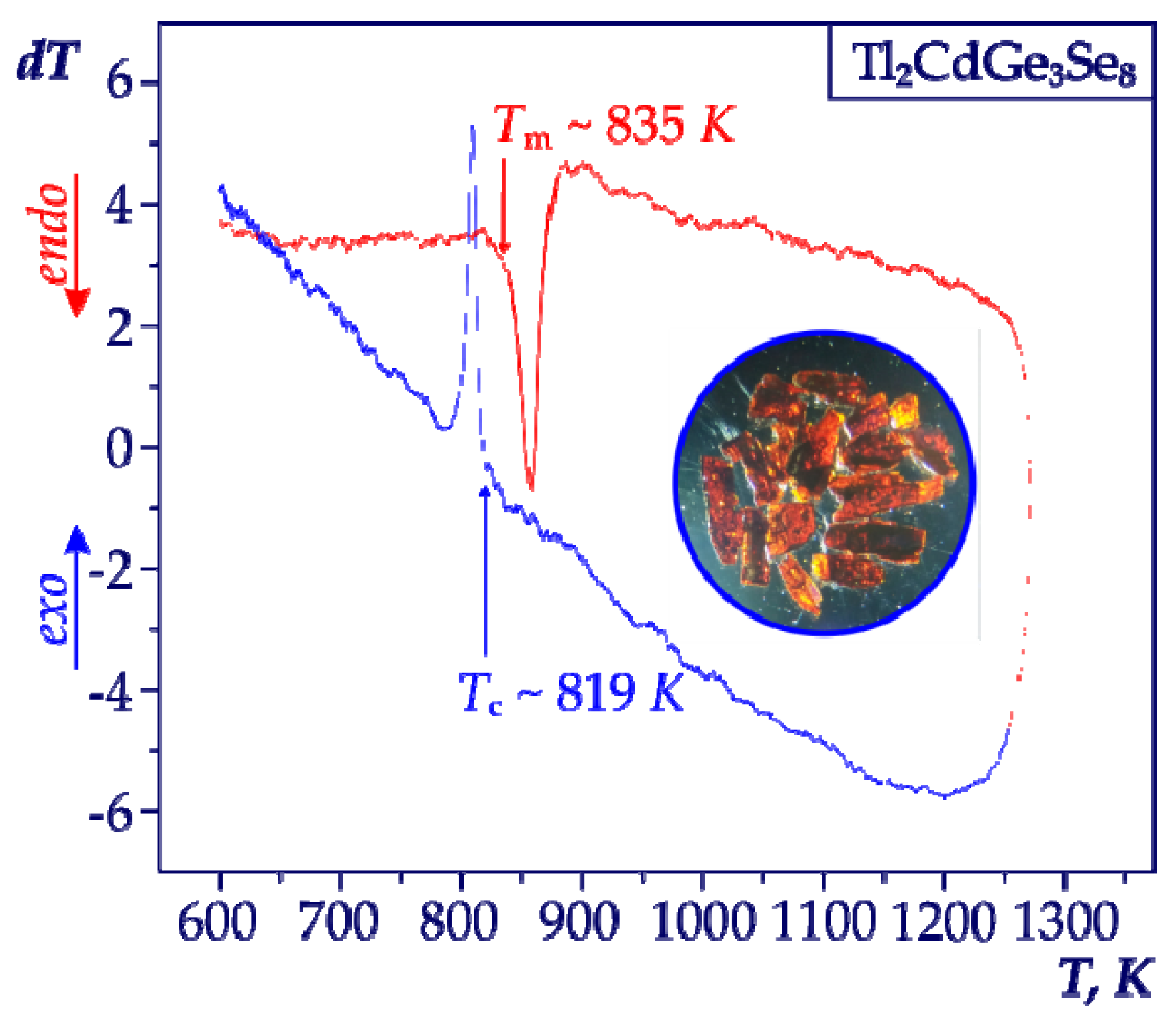
3. Results and Discussion
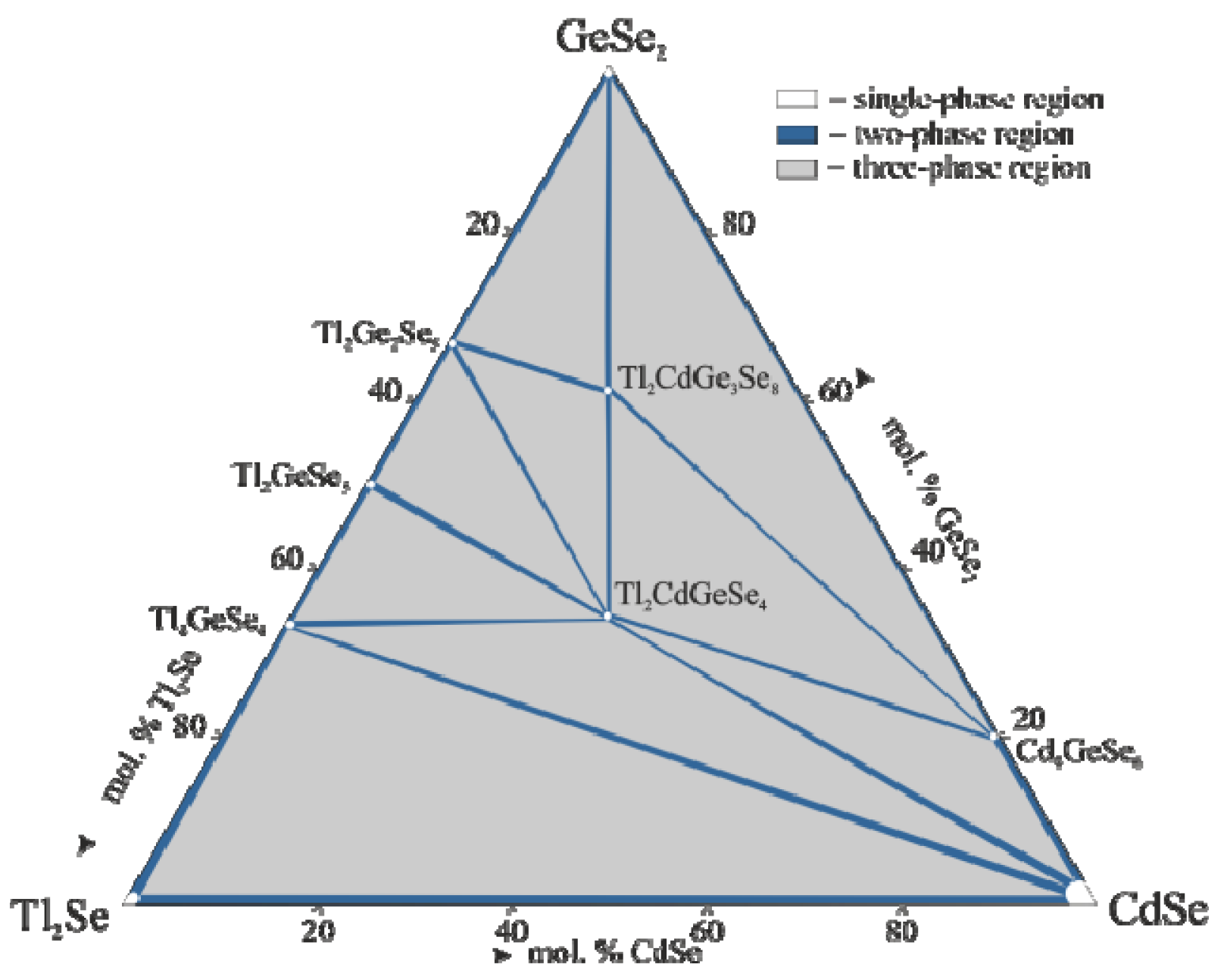
3.1. Phase Equilibria in the Tl2Se–CdSe–GeSe2 System
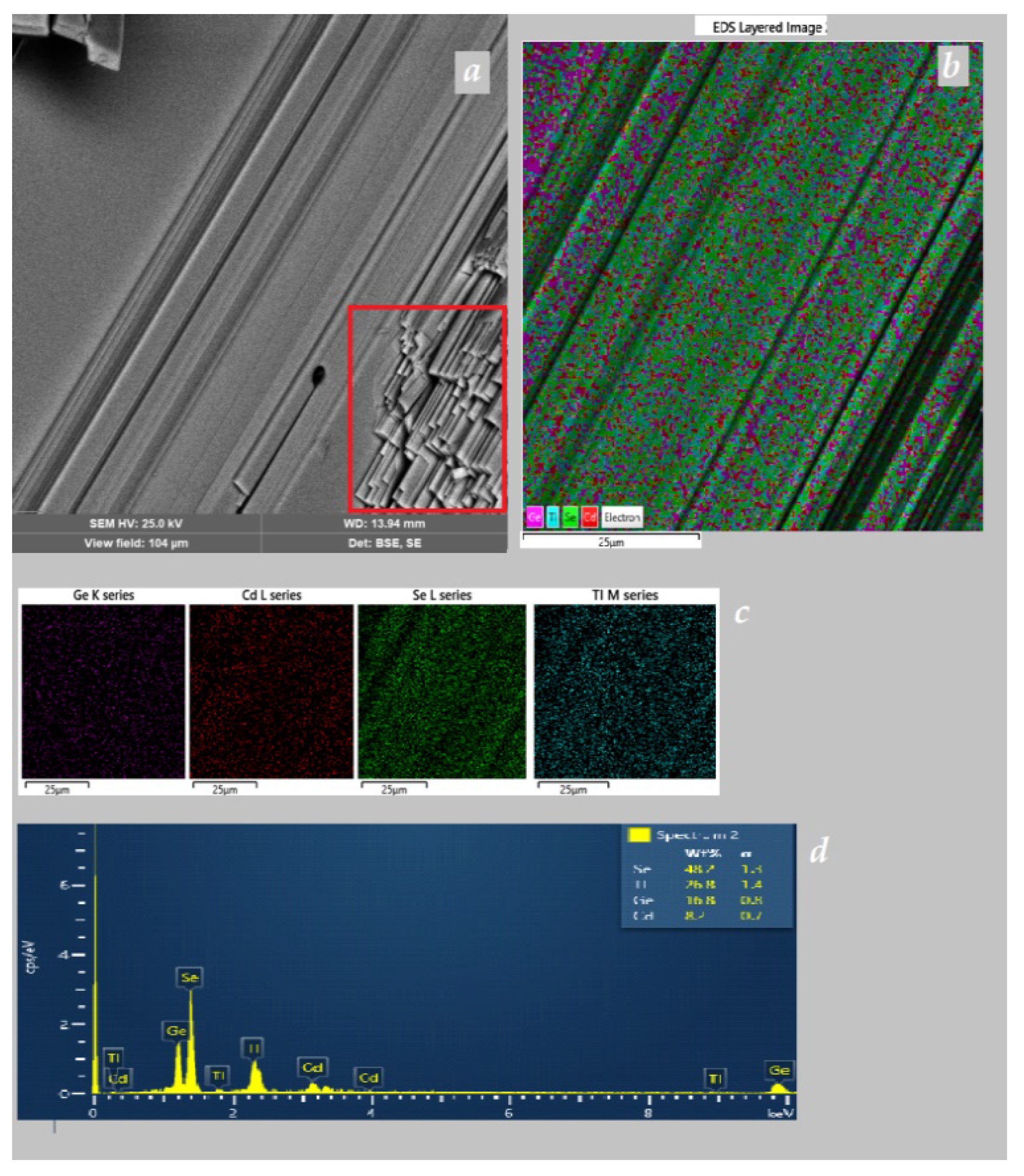
3.2. EDS Analysis
References
- McGuire, M.A.; Scheidemantel, T.J.; Badding, J.V.; DiSalvo, F.J. Tl2AXTe4 (A = Cd, Hg, Mn; X = Ge, Sn): Crystal Structure, and Thermoelectric Properties. Chem. Mater. 2005, 17, 6186–6191. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, P. Quaternary semiconductors Cu2MgSnS4 and Cu2MgSnSe4 as potential thermoelectric materials. J. Phys. Commun. 2017, 1, 045014. [Google Scholar] [CrossRef]
- Brik, M.G.; Parasyuk, O.V.; Myronchuk, G.L.; Kityk, I.V. Specific features of band structure and optical anisotropy of Cu2CdGeSe4 quaternary compounds. Mater. Chem. Phys. 2014, 147, 155–161. [Google Scholar] [CrossRef]
- Morris, C.D.; Li, H.; Jin, H.; Malliakas, C.D.; Peters, J.A.; Trikalitis, P.N.; Kanatzidis, M. GCs2MIIMIV3Q8 (Q = S, Se, Te): An Extensive Family of Layered Semiconductors with Diverse Band Gaps. Chem. Mater. 2013, 25, 3344–3356. [Google Scholar] [CrossRef]
- Fard, Z.H.; Kanatzidis, M.G. Phase-Change Materials Exhibiting Tristability: Interconverting Forms of Crystalline α-, β-, and Glassy K2ZnSn3S8. Inorg. Chem. 2012, 51, 7963–7965. [Google Scholar] [CrossRef] [PubMed]
- Pogu, A.; Vidyasagar, K. Syntheses, structural variants and characterization of A2ZnSn3S8 (A = Cs, Rb) and A2CdSn3S8 (A = Cs, Rb, K, Na) compounds. J. Solid State Chem. 2020, 291, 121647. [Google Scholar] [CrossRef]
- Chykhrij, S.I.; Sysa, L.V.; Parasyuk, O.V.; Piskach, L.V. Crystal structure of the Cu2CdSn3S8 compound. J. Alloys Compd. 2000, 307, 124–126. [Google Scholar] [CrossRef]
- Akselrud, L.G.; Grin, Y. WinCSD: software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Selezen, A.O.; Olekseyuk, I.D.; Myronchuk, G.L.; Smitiukh, O.V.; Piskach, L.V. Piskach, Synthesis and structure of the new semiconductor compounds Tl2BIIDIVX4 (BII–Cd, Hg; DIV–Si, Ge; X–Se, Te) and isothermal sections of the Tl2Se–CdSe-Ge(Sn)Se2 systems at 570 K. J. Solid State Chem. 2020, 289, 121422. [Google Scholar] [CrossRef]
| Compound | Temperature, K | Compound | Temperature, K |
|---|---|---|---|
| Tl2HgSiS4 | 654 | Tl2PbSiS4 | 818 |
| Tl2HgSiSe4 | 703 | Tl2PbSiSe4 | 788 |
| Tl2HgGeS4 | 698 | Tl2HgSnS4 | 718 |
| Tl2HgGeSe4 | 764 (congruent) | Tl2HgSnSe4 | 883 |
| Tl2PbGeS4 | 781 | Tl2CdGeSe4 | 809 |
| Tl2PbGeSe4 | 710 | Tl2CdSnSe4 | 860 |
| Parameter | Atom | Number_2 | Number_3 | Number_4 | Number_5 | Number_6 | Number_7 | Number_1_SUM |
|---|---|---|---|---|---|---|---|---|
| Tl | 26.8 | 27.3 | 27.8 | 28.7 | 29 | 27.6 | 27.8667 | |
| Wt.% | Cd | 8.2 | 8.8 | 7.7 | 9.5 | 8.3 | 9.1 | 8.6000 |
| Ge | 16.8 | 15.8 | 17 | 16.9 | 15.9 | 16.7 | 16.5167 | |
| Se | 48.2 | 48.1 | 47.5 | 44.9 | 46.7 | 46.6 | 47.0000 | |
| Tl | 1.7975 | 1.7063 | 1.9857 | 1.6616 | 1.9216 | 1.6682 | 1.7901 | |
| At.%, n | Cd | 1.0000 | 1.0001 | 1.0000 | 1.0000 | 0.9999 | 1.0000 | 1.0000 |
| Ge | 3.1717 | 2.7798 | 3.4179 | 2.7541 | 2.9656 | 2.8412 | 2.9884 | |
| Se | 8.3679 | 7.7820 | 8.7820 | 6.7287 | 8.0097 | 7.2906 | 7.8268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selezen, A.; Kogut, Y.; Piskach, L.; Gulay, L. New Quaternary Chalcogenides Tl2MIIMIV3Se8 and Tl2MIIMIVX4. Proceedings 2020, 62, 3. https://doi.org/10.3390/proceedings2020062003
Selezen A, Kogut Y, Piskach L, Gulay L. New Quaternary Chalcogenides Tl2MIIMIV3Se8 and Tl2MIIMIVX4. Proceedings. 2020; 62(1):3. https://doi.org/10.3390/proceedings2020062003
Chicago/Turabian StyleSelezen, Andrii, Yuri Kogut, Lyudmyla Piskach, and Lubomir Gulay. 2020. "New Quaternary Chalcogenides Tl2MIIMIV3Se8 and Tl2MIIMIVX4" Proceedings 62, no. 1: 3. https://doi.org/10.3390/proceedings2020062003
APA StyleSelezen, A., Kogut, Y., Piskach, L., & Gulay, L. (2020). New Quaternary Chalcogenides Tl2MIIMIV3Se8 and Tl2MIIMIVX4. Proceedings, 62(1), 3. https://doi.org/10.3390/proceedings2020062003





