Abstract
Coffee silverskin is one of the byproducts generated by the coffee industry. Although it is not the most burdensome one, because it stands only for ~4.2 wt % of coffee, it seems like an auspicious raw material for industrial processes. Coffee silverskin is characterized by a relatively low moisture content of ~5–7%, so it often does not require quite energy-consuming drying processes. The chemical composition of coffee silverskin, as well as other renewable materials, may be significantly affected by its type and origin, in this case, plant Coffea. Nevertheless, due to high fiber content, it could be considered as exciting material for the manufacturing of wood polymer composites. At the same time, it contains noticeable amounts of proteins, which may provide additional features to polymer composites. However, what is most important is the high content of antioxidants, which could noticeably enhance their lifetime by inhibition of the oxidation reactions. In the presented paper, attempts of coffee silverskin incorporation into different polymer matrices were summarized and discussed. Moreover, potential future trends in this area of research were proposed.
1. Introduction
Coffee is commonly considered as one of the most popular beverages in the world. Its consumption exceeded 165 million 60-kg bags in the crop year 2018/2019. The size of consumption is growing by ~2% annually over the last years [1], which implies increasing production, especially in South and Central America and Asia. It obviously causes increased amounts of generated byproducts. Generally, byproducts from the processing of the fresh coffee cherries into roasted coffee stand for over 50% of the initial mass of cherry [2]. The primary byproducts obtained during coffee processing are husk, pulp, mucilage, parchment, silverskin, and wastewater [3]. The coffee industry also generates enormous amounts of spent coffee grounds. The exact amounts of byproducts and their composition depend on the origin of coffee, particularly the coffee plant species. Two species, which accounts for almost 100% of coffee production are Coffea arabica (Arabica) and Coffea canephora (Robusta). The composition and amounts of byproducts are affected by climate conditions and soil, where plants are grown [3].
Another factor affecting the generation of byproducts is the method of coffee processing. Three main methods may be distinguished: natural, washed, and honey. They are aimed to produce coffee with a different flavor profile. The natural method does not use water. Cherries are dried in the sun until the whole husk is separated. This byproduct consists of the outer skin, pulp, and mucilage [4]. More complex is the washed method. The first step is the mechanical separation of coffee pulp from beans. Then, beans covered with a sticky mucilagous layer are fermented in pools, facilitating final cleaning [5]. After fermentation, beans are dried until the proper level of moisture is achieved. The honey method is a combination of natural and washed. The pulp is mechanically separated, and beans covered with mucilage are dried in the sun [6]. Independently of the selected processing method, beans have to be dried, enabling the separation of parchment—another coffee industry byproduct.
After processing and drying, the most critical step in the manufacturing of coffee is conducted—roasting. During this process, all the main chemical changes occur, directly influencing the appearance, aroma, and flavor profile of the coffee brew [7]. The caramelization process and Maillard reactions are directly affecting the color of coffee beans and the generation of melanoidins, which are natural antioxidants [8]. The only byproduct generated in the roasting process is coffee silverskin.
2. Coffee Silverskin
Coffee silverskin stands for ~4.2 wt % of coffee beans, so it is not generated in massive amounts. Nevertheless, it should be considered an auspicious material for industrial applications, including polymer composites. It is associated with its low moisture content, which makes it hardly perishable, contrary to other byproducts, especially those obtained during washed processing. The chemical composition of coffee silverskin is significantly affected by the type and origin of coffee, which can be seen in Table 1. This byproduct contains mainly carbohydrates, making it relatively similar in chemical composition to the various lignocellulose materials, such as wood flour. Coffee silverskin also contains significant amounts of proteins, which during processing may also take part in Maillard reactions, generating melanoidins enhancing the antioxidant activity of the material. Moreover, this byproduct contains noticeable amounts of other compounds showing antioxidant activity, such as caffeine, polyphenols, tannins, or melanoidins generated during roasting of coffee [9]. They are responsible for the excellent antioxidant activity of coffee silverskin, which, according to literature data, is in the range of 9–18 µmol TR/g, measured by DPPH assay [10]. The main compounds showing antioxidant activity present in coffee silverskin are summarized in Figure 1 [11,12]. Other, less popular include hyperoside, kaempferol, naringin, quercetin, or quinine.

Table 1.
Composition of the coffee silverskin according to the literature data.
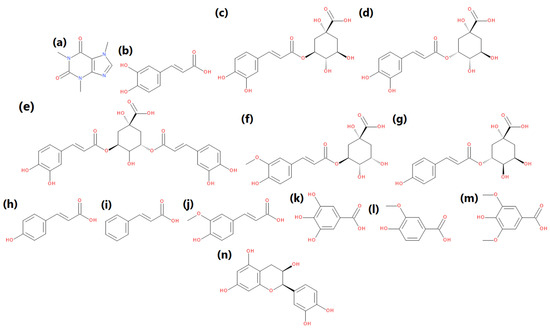
Figure 1.
The main compounds showing antioxidant activity, present in coffee silverskin: (a) caffeine, (b) caffeic acid, (c) 3-caffeoylquinic acid, (d) 5-caffeoylquinic acid, (e) 3,5-dicaffeoylquinic acid, (f) 3-feruloylquinic acid, (g) 3-p-coumaroylquinic acid, (h) p-coumaric acid, (i) trans-cynnamic acid, (j) ferulic acid, (k) gallic acid, (l) vanillic acid, (m) syringic acid, and (n) epicatechin.
Regarding the utilization of coffee silverskin, areas of interest include pyrolysis, manufacturing of biofuels or adsorbents, and biotechnological studies aimed at the production of ethanol, α-amylase, butanol, succinic acid, or mushroom growth. An exciting application is also the regeneration of frying oil, associated with polyphenols’ high content [13]. Due to the antioxidant activity, researchers are also strongly focusing on the uses related to the food and cosmetics industry [14]. Coffee silverskin and its extracts show mainly antioxidant, antiradical, anti-inflammatory, and antimicrobial activity, but researchers also reported interesting anticellulite and anti-hair loss effects [15]. Antioxidant properties are also utilized in food products, where silverskin may be introduced as a novel food ingredient, e.g., in the manufacturing of antioxidant beverages [16]. It also shows antidiabetic activity and may prevent diseases related to oxidative stress, such as diabetes. These exciting features, related to the antioxidant and antimicrobial properties of coffee silverskin, also encourage using this byproduct as a potential additive for polymeric materials.
3. Applications of Coffee Silverskin in Polymer Technology
From the technological point of view, coffee silverskin can be considered one of the most promising byproducts of the coffee industry. Due to the roasting process’s characteristics, it is characterized by the very low moisture content compared to other byproducts. Silverskin does not require quite energy-consuming drying processes, which beneficially affects the economic issues. Due to the minimal number of coffee silverskin industrial applications, it is treated as waste by the roasteries. Therefore, to reduce its volume, it is often pelletized or compacted after mixing with water or oil, which are aimed to act as binders. Such treatment significantly affects the most significant advantage of silverskin, the low moisture content. Therefore, it is justified to develop the applications for coffee silverskin, which would provide it added value. Among the potential examples, incorporation of this material to the polymer technology seems very promising.
As mentioned above, coffee silverskin shows quite similar composition to materials conventionally applied as fillers for wood polymer composites. It usually contains about 60–70% of fiber, mostly insoluble (~90%). What distinguishes this byproduct is the relatively high protein content (~17–18%), which may provide additional polymer composites features. They can act as plasticizers of the polymer matrix, and as mentioned earlier, take part in Maillard reactions. Moreover, due to functional groups’ presence, proteins could provide additional possibilities for interfacial adhesion with polar polymer matrices. Thanks to the high content of antioxidants, silverskin may be beneficial for polymer composites’ storage stability. Nevertheless, the use of this byproduct in polymer technology still focuses mainly on mechanical performance without a more in-depth analysis of the additional effects. The reported effects of the coffee silverskin incorporation into polymer composites on their mechanical performance are summarized in Table 2.

Table 2.
The impact of coffee silverskin on the mechanical performance of polymer composites.
Zarrinbakhsh et al. [17] compared coffee silverskin with the other coffee industry byproduct—spent coffee grounds. They introduced 25 wt % of fillers into the polypropylene matrix. Spent coffee grounds showed twice as higher lipid content comparing to silverskin. Moreover, both fillers showed different morphology, silverskin particles were flake-shaped with fibrous structures, while spent coffee grounds were mostly in the form of irregular-shape particles. Properties of composites were strongly affected by the differences between fillers. In both cases, insufficient interfacial adhesion was noted, which was related to the significant differences in phases’ polarity. Nevertheless, the presence of silverskin caused over 30% enhancement of tensile and flexural modulus. The authors indicated the need for compatibilization of composites containing silverskin, which was confirmed later by other researchers [34].
Dominici et al. [35] used this hint and performed alkali treatment and esterification with palmitoyl chloride before introducing silverskin into the polyethylene matrix. It caused enhancement of elongation at the break because delamination during the tensile test was delayed. This work confirmed the need for enhancement of the interfacial interactions.
Except for polyolefin matrices, coffee silverskin was mostly introduced into biodegradable polymers, which should be considered environmentally friendly. Totaro et al. [33] applied poly(lactic acid) (PLA) and poly(butylene succinate) (PBS) as matrices for coffee silverskin composites. No additional modifications of filler were applied. Similarly to other works [31,32,33], a noticeable increase in tensile modulus was observed, associated with over 40% increase in the degree of crystallinity for both matrices (up to 82% for PLA). Considering tensile strength, deterioration was noted for both matrices. However, it was more pronounced for PLA, which was related to its exceptional strength and higher processing temperature (190 °C, while for PBS was 125 °C). At elevated temperature, decomposition of PLA occurred, which was catalyzed by metal ions present in silverskin.
Biodegradable matrix was also applied for composites filled with coffee silverskin by Sarasini et al. [31], who used poly(butylene adipate-co-terephthalate)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PBAT/PHBV) blend. The authors used two types of silverskin, untreated and sieved through 150 µm sieve. The application of silverskin with the smaller particle size caused the enhancement of the mechanical performance, compared to the untreated byproduct (see Table 2). Such an effect was related to the higher interface surface area, as suggested by the SEM analysis. The byproduct, independent of the particle size, showed antioxidant activity of 16.06 mg TE/g of dry weight, determined by DPPH assay. It was associated with phenolics’ content and resulted in over nine-fold elongation of the oxidation induction time compared to the neat PBAT/PHBV blend. The authors also prepared methanol extracts of composite samples. They showed over 90% DPPH scavenging activity, while for the neat blend, its value was 0.
In their other work, Sarasini et al. [32] investigated the compatibilization of the above-mentioned PBAT/PHBV/CSS composites. The authors introduced maleinized linseed oil (MLO) and (3-aminopropyl)triethoxysilane (APTES). The silverskin was used as received, without grinding and sieving, and after the extraction of antioxidants in water. As a result, compatibility with PBAT/PHBV matrix was enhanced, attributed to the removal of water-soluble components and the rougher surface of fillers. Filler pretreatment resulted in an even 10% increase of tensile strength and over 30% enhancement of composite stiffness. All of the applied compatibilization methods increased tensile strength comparing to the neat polymer matrix. The best results were noted for water extraction, relatively similar to silanization, and significantly higher than for the addition of maleinized linseed oil. Similar trends were noted for Young’s modulus, while elongation at break was significantly decreased for all composite samples.
4. Conclusions
The presented paper summarized the literature reports related to the chemical composition of coffee silverskin and its methods of utilization in polymer technology reported in the literature. As presented in this work, based on the chemical composition, coffee silverskin may act as filler for wood polymer composites and as a stabilizer, enhancing their resistance towards oxidation and different types of microorganisms. It may be directly incorporated into polymer matrices or used as the source of particular compounds applied as modifiers. There are hardly any reports related to the antioxidant activity of coffee silverskin in polymer-based materials. Future research works should focus on the following issues:
- in-depth investigation of the mechanisms of action of particular antioxidants present in the coffee silverskin in terms of the potential enhancement of the oxidative stability of different polymeric materials,
- comprehensive analysis of the antimicrobial activity of coffee silverskin and its impact on the stability of polymeric materials,
- possibility for the engineering of materials with the desired rate of decomposition under different conditions.
Author Contributions
A.H. analyzed the data; A.H. wrote the paper.
Conflicts of Interest
The author declares no conflict of interest.
References
- Statista.com. Coffee Consumption Worldwide from 2012/13 to 2018/19 (in Million 60 kg Bags). Available online: https://www.statista.com/statistics/292595/global-coffee-consumption/ (accessed on 10 July 2020).
- Adams, M.R.; Dougan, J. Waste Products. In Coffee; Clarke, R.J., Macrae, R., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 257–291. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Taveira, J.H.D.S.; Sttela, D.V.F.D.R.; Pedro, D.O.; Gerson, S.G.; Eder, P.I. Post-harvest effects on beverage quality and physiological performance of coffee beans. Afr. J. Agric. Res. 2015, 10, 1457–1466. [Google Scholar] [CrossRef]
- Agate, A.D.; Bhat, J.V. Role of pectinolytic yeasts in the degradation of mucilage layer of Coffea robusta cherries. Appl. Environ. Microb. 1966, 14, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.C.; Calado, V.; Franca, A.S.; Trugo, L.C. Correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Arya, M.; Rao, L.J. An impression of coffee carbohydrates. Crit. Rev. Food Sci. 2007, 47, 51–67. [Google Scholar] [CrossRef]
- Cämmerer, B.; Kroh, L.W. Antioxidant activity of coffee brews. Eur. Food Res. Technol. 2006, 223, 469–474. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Conde, T.; Mussatto, S.I. Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep. Biochem. Biotechnol. 2015, 46, 406–409. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Ismail, S.A.A.; El-Anany, A.M.; Ali, R.F.M. Regeneration of Used Frying Palm Oil with Coffee Silverskin (CS), CS Ash (CSA) and Nanoparticles of CS (NCS). J. Oleo Sci. 2017, 66, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Wang, T.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Characterization of wastes and coproducts from the coffee industry for composite material production. BioResources 2016, 11, 7637–7653. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recy. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Behrouzian, F.; Amini, A.M.; Alghooneh, A.; Razavi, S.M.A. Characterization of dietary fiber from coffee silverskin: An optimization study using response surface methodology. Bioact. Carbohyd. Diet. Fibre 2016, 8, 58–64. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee Silverskin: Characterization, Possible Uses, and Safety Aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Napolitano, A.; Fogliano, V.; Tafuri, A.; Ritieni, A. Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 2007, 55, 10499–10504. [Google Scholar] [CrossRef] [PubMed]
- Niglio, S.; Procentese, A.; Russo, M.; Sannia, G.; Marzocchella, A. Investigation of Enzymatic Hydrolysis of Coffee Silverskin Aimed at the Production of Butanol and Succinic Acid by Fermentative Processes. BioEnergy Res. 2019, 12, 312–324. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. J. Food Meas. Charact. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Diez-Antolinez, R. Biobutanol production from coffee silverskin. Microb. Cell Fact. 2019, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Niglio, S.; Procentese, A.; Russo, M.; Sannia, G.; Marzocchella, A. Ultrasound-assisted Dilute Acid Pretreatment of Coffee Silverskin for Biorefinery Applications. Chem. Eng. Trans. 2017, 57, 109–114. [Google Scholar] [CrossRef]
- Sánchez, D.A.; Anzola, C. Caracterización química de la película plateada del café (Coffea arábica) en variedades colombia y caturra. Rev. Colomb. Quím. 2012, 41, 211–226. [Google Scholar]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Sarasini, F.; Tirillò, J.; Zuorro, A.; Maffei, G.; Lavecchia, R.; Puglia, D.; Dominici, F.; Luzi, F.; Valente, T.; Torre, L. Recycling coffee silverskin in sustainable composites based on a poly(butylene adipate-co-terephthalate)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrix. Ind. Crop. Prod. 2018, 118, 311–320. [Google Scholar] [CrossRef]
- Sarasini, F.; Luzi, F.; Dominici, F.; Maffei, G.; Iannone, A.; Zuorro, A.; Lavecchia, R.; Torre, L.; Carbonell-Verdu, A.; Balart, R.; et al. Effect of Different Compatibilizers on Sustainable Composites Based on a PHBV/PBAT Matrix Filled with Coffee Silverskin. Polymers 2018, 10, 1256. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Fiorini, M.; Lancellotti, I.; Andreola, F.N.; Saccani, A. Formulation of Green Particulate Composites from PLA and PBS Matrix and Wastes Deriving from the Coffee Production. J. Polym. Environ. 2019, 27, 1488–1496. [Google Scholar] [CrossRef]
- Ochoa, D.; Rojas-Vargas, J.; Costa, Y. Characterization of NaOH-Treated Colombian Silverskin Coffee Fiber as a Composite Reinforcement. BioResources 2017, 12, 8803–8812. [Google Scholar]
- Dominici, F.; García García, D.; Fombuena, V.; Luzi, F.; Puglia, D.; Torre, L.; Balart, R. Bio-Polyethylene-Based Composites Reinforced with Alkali and Palmitoyl Chloride-Treated Coffee Silverskin. Molecules 2019, 24, 3113. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).