Polymeric Flavonoids Obtained by Crosslinking Reaction †
Abstract
:1. Introduction
2. Experiments
2.1. Polymerization
2.2. Differential Scanning Calorimetry (DSC)
2.3. FRAP and CUPRAC Analysis
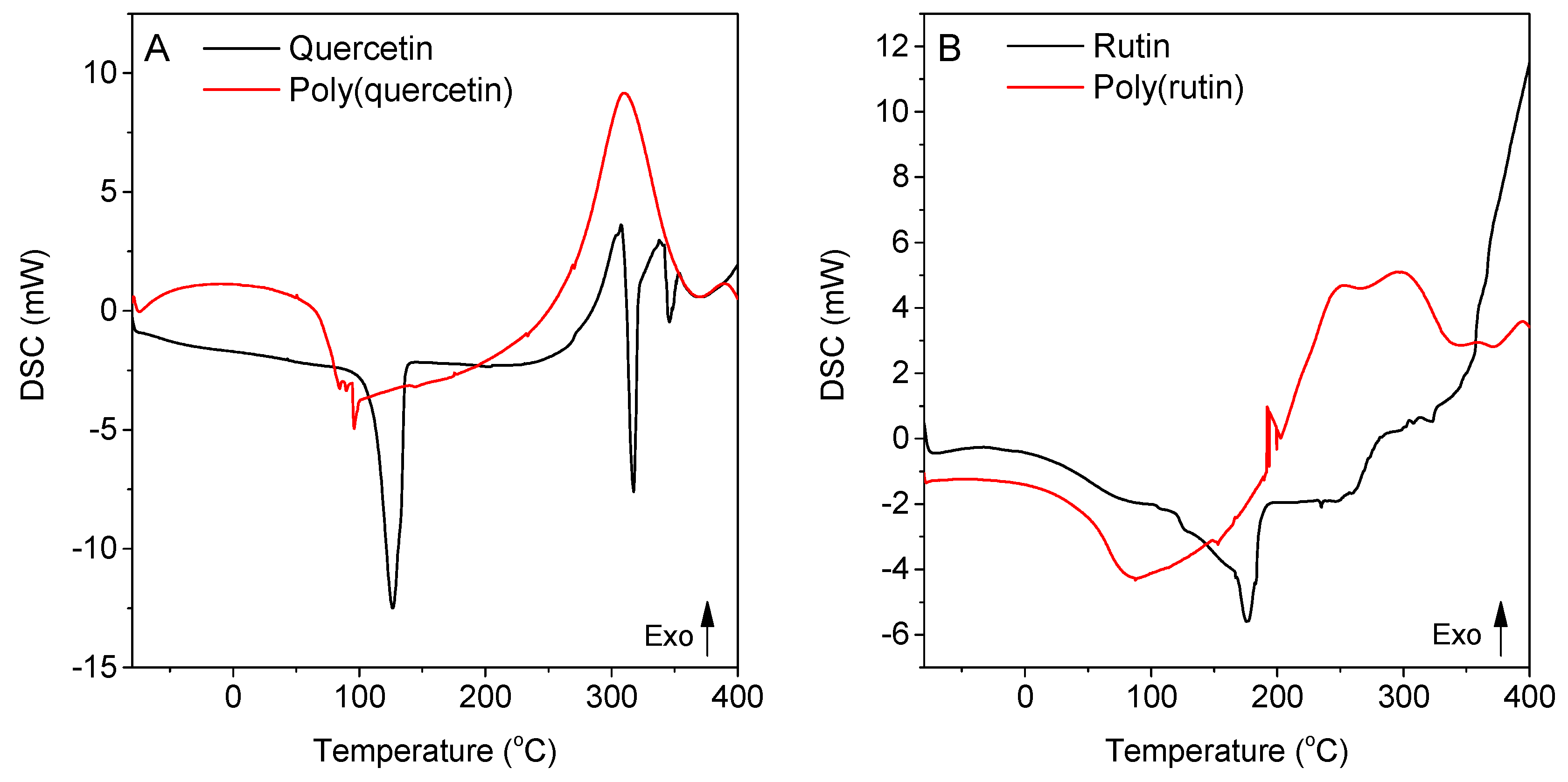
3. Results and Discussion
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSC | Differential Scanning Calorimetry |
| CUPRAC | Cupric Reducing Antioxidant Capacity |
| FRAP | Ferric Reducing Antioxidant Power |
| GDE | glycerol diglycdyl ether |
References
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Gullon, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure-Activity Relationship of Flavonoids on Their Anti-Escherichia coli Activity and Inhibition of DNA Gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Jacob, V.; Hagai, T.; Soliman, K. Structure-activity relationships of flawonoids. Curr. Org. Chem. 2011, 15, 2641–2657. [Google Scholar] [CrossRef]
- Sahiner, N. One step poly(quercetin) particle preparation as biocolloidand its characterization. Coll. Surf. A Physicochem. Eng. Asp. 2014, 452, 173–180. [Google Scholar] [CrossRef]
- Sahiner, N. One step poly(rutin) particle preparation as biocolloid and its characterization. Mater. Sci. Eng. C 2014, 44, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Latos-Brozio, M.; Masek, A. Biodegradable Polyester Materials Containing Gallates. Polymers 2020, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Latos-Brozio, M.; Kałużna-Czaplińska, J.; Rosiak, A.; Chrześcijańska, E. Antioxidant Properties of Green Coffee Extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]

| Sample | ΔHm [J/g] | Tm [°C] | ΔHo [J/g] | To [°C] |
|---|---|---|---|---|
| Quercetin | 193.0 | 111.0 | 99.9 | 312.4 (endset) |
| Poly(quercetin) | 119.2 | 48.6 | 626.5 | 351.6 (endset) |
| Rutin | 115.7 | 160.3 | 31.3 | 313.3 (endset) |
| Poly(rutin) | 118.3 | 52.4 | 460.4 | 258.5 (endset) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latos-Brozio, M.; Masek, A. Polymeric Flavonoids Obtained by Crosslinking Reaction. Proceedings 2021, 69, 27. https://doi.org/10.3390/CGPM2020-07194
Latos-Brozio M, Masek A. Polymeric Flavonoids Obtained by Crosslinking Reaction. Proceedings. 2021; 69(1):27. https://doi.org/10.3390/CGPM2020-07194
Chicago/Turabian StyleLatos-Brozio, Malgorzata, and Anna Masek. 2021. "Polymeric Flavonoids Obtained by Crosslinking Reaction" Proceedings 69, no. 1: 27. https://doi.org/10.3390/CGPM2020-07194
APA StyleLatos-Brozio, M., & Masek, A. (2021). Polymeric Flavonoids Obtained by Crosslinking Reaction. Proceedings, 69(1), 27. https://doi.org/10.3390/CGPM2020-07194






