Modification of Ca2+-Crosslinked Sodium Alginate/Gelatin Films with Propolis for an Improved Antimicrobial Action †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. MICs
2.3. Polymeric Films Production
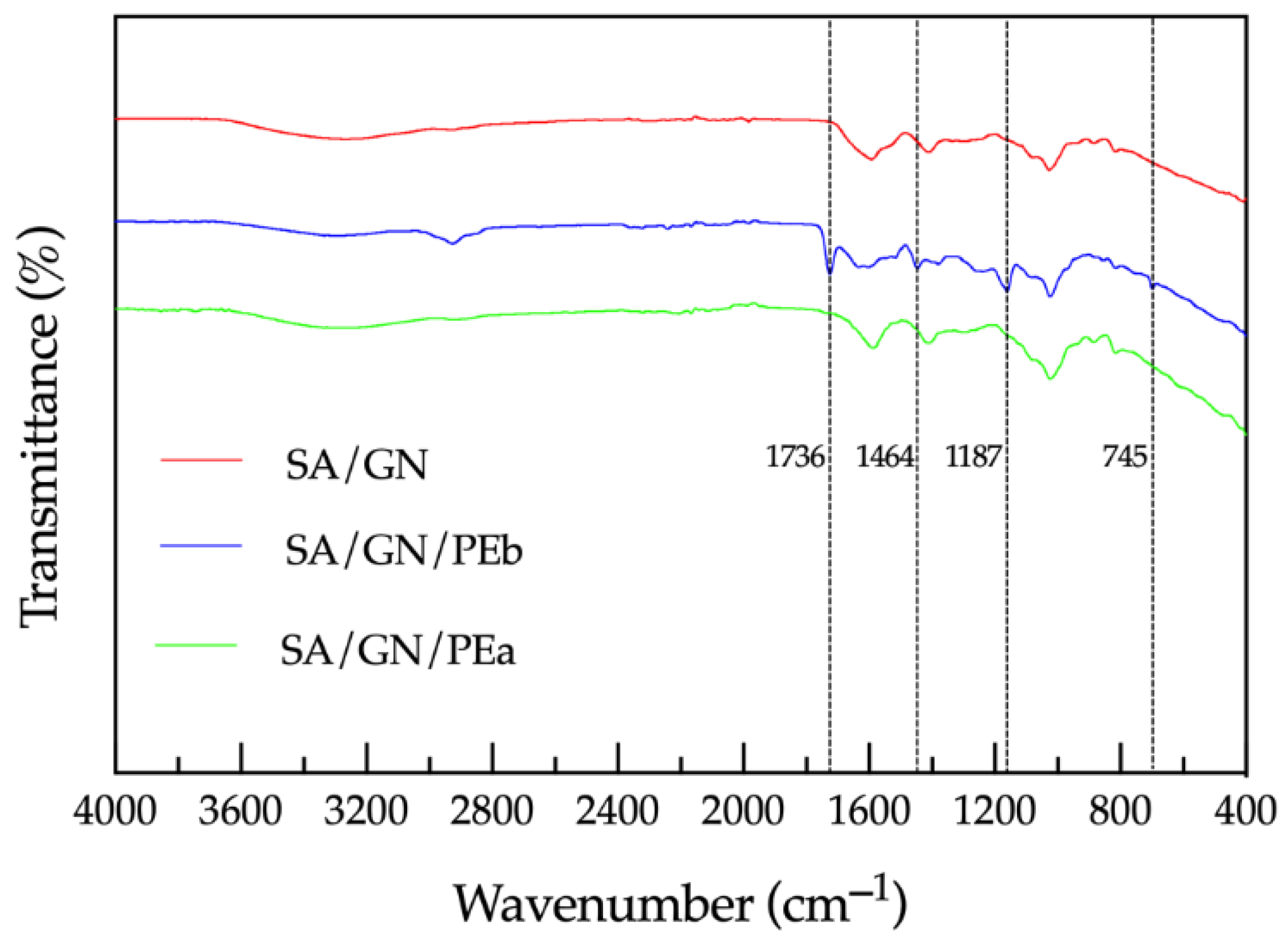
2.4. Films Characterization via ATR-FTIR
2.5. Water Absorption and Retention Capacity of SA/GN Films
2.6. Agar Disk Diffusion Tests
2.7. Killing Time Kinetics
3. Results and Discussion
3.1. MICs
3.2. Films Characterization via ATR-FTIR
3.3. Water Absorption and Retention Properties of SA/GN Films
3.4. Agar Disk Diffusion Tests
3.5. Killing Time Kinetics
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.M.; Antunes, J.C.; Amorim, M.T.S.P. Physical, Thermal, and Antibacterial Effects of Active Essential Oils with Potential for Biomedical Applications Loaded onto Cellulose Acetate/Polycaprolactone Wet-Spun Microfibers. Biomology 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Beluci, N.D.C.L.; Homem, N.C.; Amorim, M.T.S.P.; Bergamasco, R.; Vieira, A.M.S. Biopolymer extracted from Moringa oleifera Lam. in conjunction with graphene oxide to modify membrane surfaces. Environ. Technol. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Bosio, K.; Avanzini, C.; D’Avolio, A.; Ozino, O.; Savoia, D. In vitro activity of propolis against Streptococcus pyogenes. Lett. Appl. Microbiol. 2000, 31, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Pala, C.; Cossu, M. Preparation and characterisation of polymeric films containing propolis. J. Drug Deliv. Sci. Technol. 2007, 17, 177–181. [Google Scholar] [CrossRef]
- Rufatto, L.C.; Dos Santos, D.A.; Marinho, F.; Henriques, J.A.P.; Ely, M.R.; Moura, S. Red propolis: Chemical composition and pharmacological activity. Asian Pac. J. Trop. Biomed. 2017, 7, 591–598. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Homem, N.C.; Zille, A.; Amorim, M.T.P. Antimicrobial action and clotting time of thin, hydrated poly(vinyl alcohol)/cellulose acetate films functionalized with LL37 for prospective wound-healing applications. J. Appl. Polym. Sci. 2019, 137, 48626. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ribeiro, A.R.M.; Homem, N.C.; Felgueiras, H.P. Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Baldevraj, R.M.; Jagadish, R. Incorporation of chemical antimicrobial agents into polymeric films for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier BV: Amsterdam, The Netherlands, 2011; pp. 368–420. [Google Scholar]
- Saravanan, M.; Rao, K.P. Pectin–gelatin and alginate–gelatin complex coacervation for controlled drug delivery: Influence of anionic polysaccharides and drugs being encapsulated on physicochemical properties of microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Zovko-Končić, M.; Kosalec, I. Modulation of antioxidant, chelating and antimicrobial activity of poplar chemo-type propolis by extraction procures. LWT Food Sci. Technol. 2014, 57, 530–537. [Google Scholar] [CrossRef]
- Devi, N.; Kakati, D.K. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J. Food Eng. 2013, 117, 193–204. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; da Silva, P.F.; Azevedo, L.F.; da Rocha, L.G.; de Moraes Porto, I.C.C.; e Moura, T.F.A.L.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; da Silva Fonseca, E.J.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean Propolis from the Adriatic Sea Islands as a Source of Natural Antioxidants: Comprehensive Chemical Biodiversity Determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP Assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Ahi, Z.B.; Renkler, N.Z.; Seker, M.G.; Tuzlakoglu, K. Biodegradable Polymer Films with a Natural Antibacterial Extract as Novel Periodontal Barrier Membranes. Int. J. Biomater. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]


| Sample | Water Retention (%) | Thickness (mm) |
|---|---|---|
| SA/GN | 2704.82 | 1.908 |
| SA/GN/PEb | 1776.33 | 1.095 |
| SA/GN/PEa | 2954.54 | 0.605 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homem, N.C.; Miranda, C.A.F.d.S.; Antunes, J.I.d.C.; de Amorim, M.T.S.P.; Felgueiras, H.P. Modification of Ca2+-Crosslinked Sodium Alginate/Gelatin Films with Propolis for an Improved Antimicrobial Action. Proceedings 2021, 69, 4. https://doi.org/10.3390/CGPM2020-07180
Homem NC, Miranda CAFdS, Antunes JIdC, de Amorim MTSP, Felgueiras HP. Modification of Ca2+-Crosslinked Sodium Alginate/Gelatin Films with Propolis for an Improved Antimicrobial Action. Proceedings. 2021; 69(1):4. https://doi.org/10.3390/CGPM2020-07180
Chicago/Turabian StyleHomem, Natália Cândido, Catarina Alexandra Fortuna dos Santos Miranda, Joana Isabel da Costa Antunes, Maria Teresa Sousa Pessoa de Amorim, and Helena Prado Felgueiras. 2021. "Modification of Ca2+-Crosslinked Sodium Alginate/Gelatin Films with Propolis for an Improved Antimicrobial Action" Proceedings 69, no. 1: 4. https://doi.org/10.3390/CGPM2020-07180









