Dual/Bi-Stage Curing of Nanocomposites from Renewable Resources upon Volumetric Expansion †
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Methods
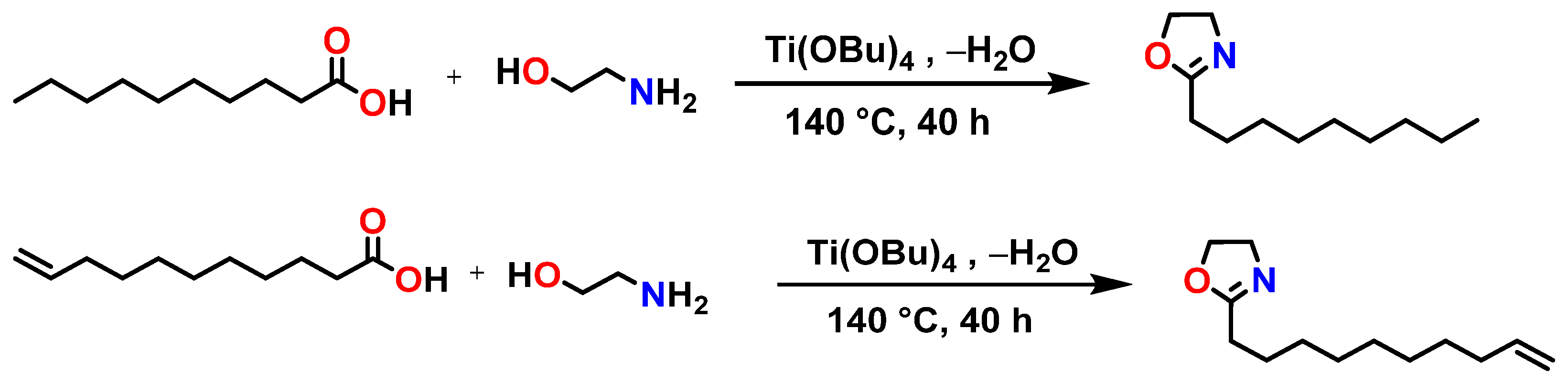
2.2. Synthesis of the Monomers Dec=Ox, NonOx, and the SOE
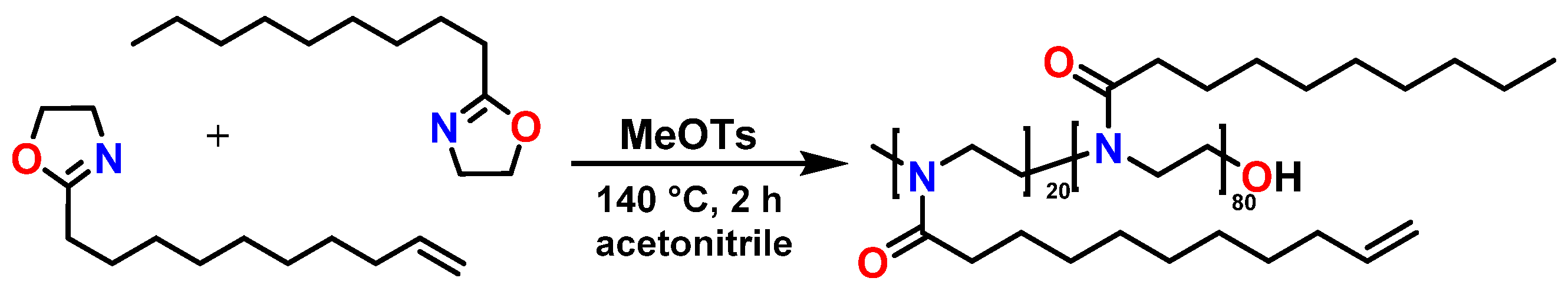
2.3. Synthesis of the Copolymer Poly(2-nonyl-2-oxazoline)-stat-poly(2-dec-9′-enyl-2-oxazoline)
2.4. Preparation of the Test Specimens
3. Results and Discussion
3.1. Copolymer Synthesis
3.2. Library Design
3.3. Determination of the Volumetric Expansion
3.4. Dielectric Properties of the Polymer Networks and the Corresponding Composites
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AlN | aluminum nitride |
| Al2O3 | aluminum oxide |
| BN | boron nitride |
| CROP | cationic ring-opening polymerization |
| DCP | dicumyl peroxide |
| DoE | design of experiments |
| Dec=Ox | 2-dec-9′-enyl-oxazoline |
| hBN | hexagonal boron nitride |
| MeOTs | methyl tosylate |
| NonOx | 2-nonyl-oxazoline |
| PA | polyamide |
| pNonOx80-stat-pDec=Ox20 | poly(2-nonyl-2-oxazoline)-stat-poly(2-dec-9′-enyl-2-oxazoline) |
| POx | poly(2-oxazoline) |
| SOE | spiroorthoester |
| wt.-% | weight percent |
References
- Sato, K.; Horibe, H.; Shirai, T.; Hotta, Y.; Nakano, H.; Nagai, H.; Mitsuishi, K.; Wataria, K. Thermally conductive composite films of hexagonal boron nitride and polyimide with affinity-enhanced interfaces. J. Mater. Chem. 2010, 20, 2749–2752. [Google Scholar] [CrossRef]
- Marx, P.; Wanner, A.J.; Zhang, Z.; Jin, H.; Tsekmes, I.-A.; Smit, J.J.; Kern, W.; Wiesbrock, F. Effect of Interfacial Polarization and Water Absorption on the Dielectric Properties of Epoxy-Nanocomposites. Polymers 2017, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J. Thermal conductivity of epoxy composites with a binaryparticle system of aluminum oxide and aluminum nitride fillers. Compos. Part B Eng. 2013, 51, 140–147. [Google Scholar] [CrossRef]
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, microcracks and fracture in polymer structures: Formation, detection, autonomic repair. Progr. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Fimberger, M.; Tsekmes, I.A.; Kochetov, R.; Smit, J.J.; Wiesbrock, F. Crosslinked poly(2-oxazoline)s as ‘green’ materials for electronic applications. Polymers 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Birnbrich, P.; Eicken, U.; Fischer, H.; Fristad, W.E.; Hase, B.; Krause, H.-J. Polyoxazoline auf fettchemischer Basis. Angew. Makromol. Chem. 1994, 223, 217–233. [Google Scholar] [CrossRef]
- Marx, P.; Romano, A.; Fischer, R.; Roppolo, I.; Sangermano, M.; Wiesbrock, F. Dual-Cure Coatings: Spiroorthoesters as Volume-Controlling Additives in Thiol-Ene Reactions. Macromol. Mater. Eng. 2019, 304, 1800627. [Google Scholar] [CrossRef]

| SOE wt.-% | No Particles: ρ (g·cm−3) | hBN, 40 wt.-%: ρ (g·cm−3) | n-AlN/μ-AIN, 20/20 wt.-%: ρ (g·cm−3) | hBN/n-AlN/μ-AIN, 20/10/10 wt.-%: ρ (g·cm−3) |
|---|---|---|---|---|
| 0 | 1.05 | 1.23 | 1.29 | 1.29 |
| 15 | 1.04 | 1.21 | 1.24 | 1.23 |
| 30 | 1.01 | 1.17 | 1.19 | 1.19 |
| SOE wt.-% | No Particles: ρ (g·cm−3) | hBN, 40 wt.-%: ρ (g·cm−3) | n-AlN/μ-AIN, 20/20 wt.-%: ρ (g·cm−3) | hBN/n-AlN/μ-AIN, 20/10/10 wt.-%: ρ (g·cm−3) |
|---|---|---|---|---|
| 0 | 1.02 | 1.21 | 1.26 | 1.28 |
| 15 | 1.01 | 1.20 | 1.25 | 1.27 |
| 30 | 0.99 | 1.19 | 1.24 | 1.26 |
| SOE wt.-% | No Particles: ΔVrel (%) | hBN, 40 wt.-%: ΔVrel (%) | n-AlN/μ-AlN, 20/20 wt.-% ΔVrel (%) | hBN/n-AlN/μ-AlN, 20/10/10 wt.-% ΔVrel (%) |
|---|---|---|---|---|
| 0 | - | - | - | - |
| 15 | 1.54 | 1.39 | 3.70 | 4.97 |
| 30 | 4.39 | 4.84 | 7.69 | 7.50 |
| SOE wt.-% | No Particles: ΔVrel (%) | hBN, 40 wt.-%: ΔVrel (%) | n-AlN/μ-AlN, 20/20 wt.-% ΔVrel (%) | hBN/n-AlN/μ-AlN, 20/10/10 wt.-% ΔVrel (%) |
|---|---|---|---|---|
| 0 | - | - | - | - |
| 15 | 0.98 | 0.84 | 0.46 | 0.84 |
| 30 | 2.48 | 1.41 | 1.92 | 1.60 |
| SOE wt.-% | No Particles: tanδ | hBN, 40 wt.-%: tanδ | n-AlN/μ-AlN, 20/20 wt.-%: tanδ | hBN/n-AlN/μ-AlN, 20/10/10 wt.-%: tanδ |
|---|---|---|---|---|
| 0 | 0.10 | 0.09 | 0.11 | 0.16 |
| 15 | 0.16 | 0.18 | 0.25 | 0.46 |
| 30 | 0.17 | 0.44 | 0.18 | 0.26 |
| SOE wt.-% | No Particles: tanδ | hBN, 40 wt.-%: tanδ | n-AlN/μ-AlN, 20/20 wt.-%: tanδ | hBN/n-AlN/μ-AlN, 20/10/10 wt.-%: tanδ |
|---|---|---|---|---|
| 0 | 0.10 | 0.07 | 0.07 | 0.07 |
| 15 | 0.24 | 0.24 | 0.15 | 0.12 |
| 30 | 0.14 | 0.13 | 0.13 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaschke, F.; Marx, P.; Wiesbrock, F. Dual/Bi-Stage Curing of Nanocomposites from Renewable Resources upon Volumetric Expansion. Proceedings 2021, 69, 3. https://doi.org/10.3390/CGPM2020-07161
Blaschke F, Marx P, Wiesbrock F. Dual/Bi-Stage Curing of Nanocomposites from Renewable Resources upon Volumetric Expansion. Proceedings. 2021; 69(1):3. https://doi.org/10.3390/CGPM2020-07161
Chicago/Turabian StyleBlaschke, Fabio, Philipp Marx, and Frank Wiesbrock. 2021. "Dual/Bi-Stage Curing of Nanocomposites from Renewable Resources upon Volumetric Expansion" Proceedings 69, no. 1: 3. https://doi.org/10.3390/CGPM2020-07161
APA StyleBlaschke, F., Marx, P., & Wiesbrock, F. (2021). Dual/Bi-Stage Curing of Nanocomposites from Renewable Resources upon Volumetric Expansion. Proceedings, 69(1), 3. https://doi.org/10.3390/CGPM2020-07161






