Can Minerals Be Used as a Tool to Classify Cinnamon Samples? †
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Mineral Determination
2.2.3. Sample Classification
3. Results and Discussion
3.1. General Information about Cinnamon Samples
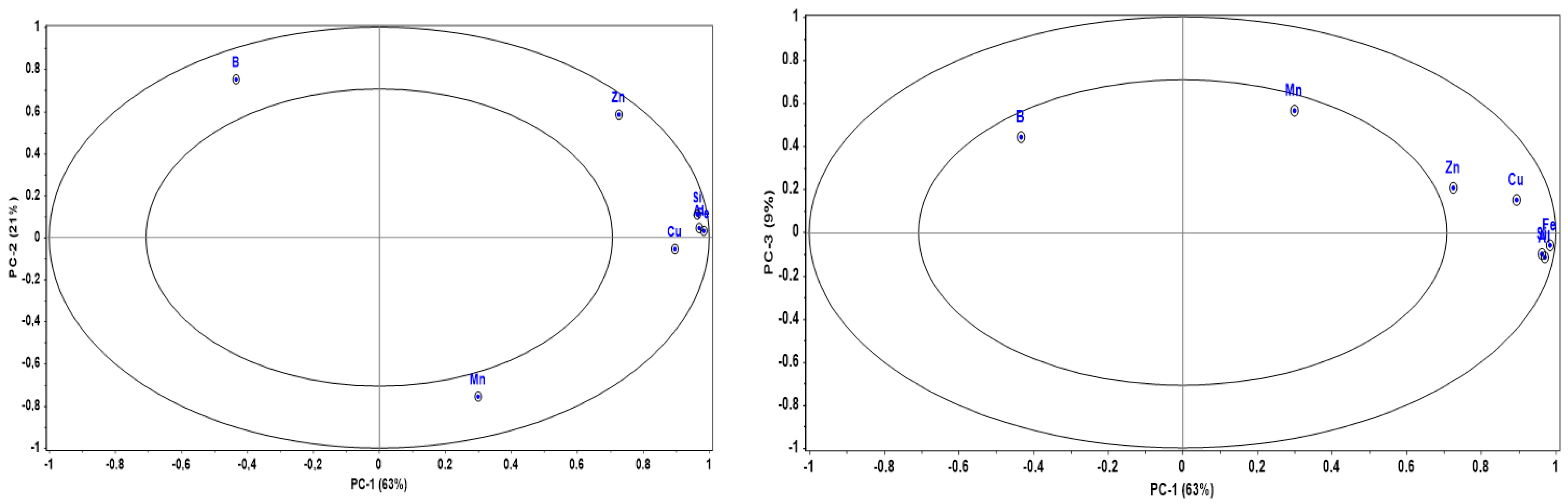
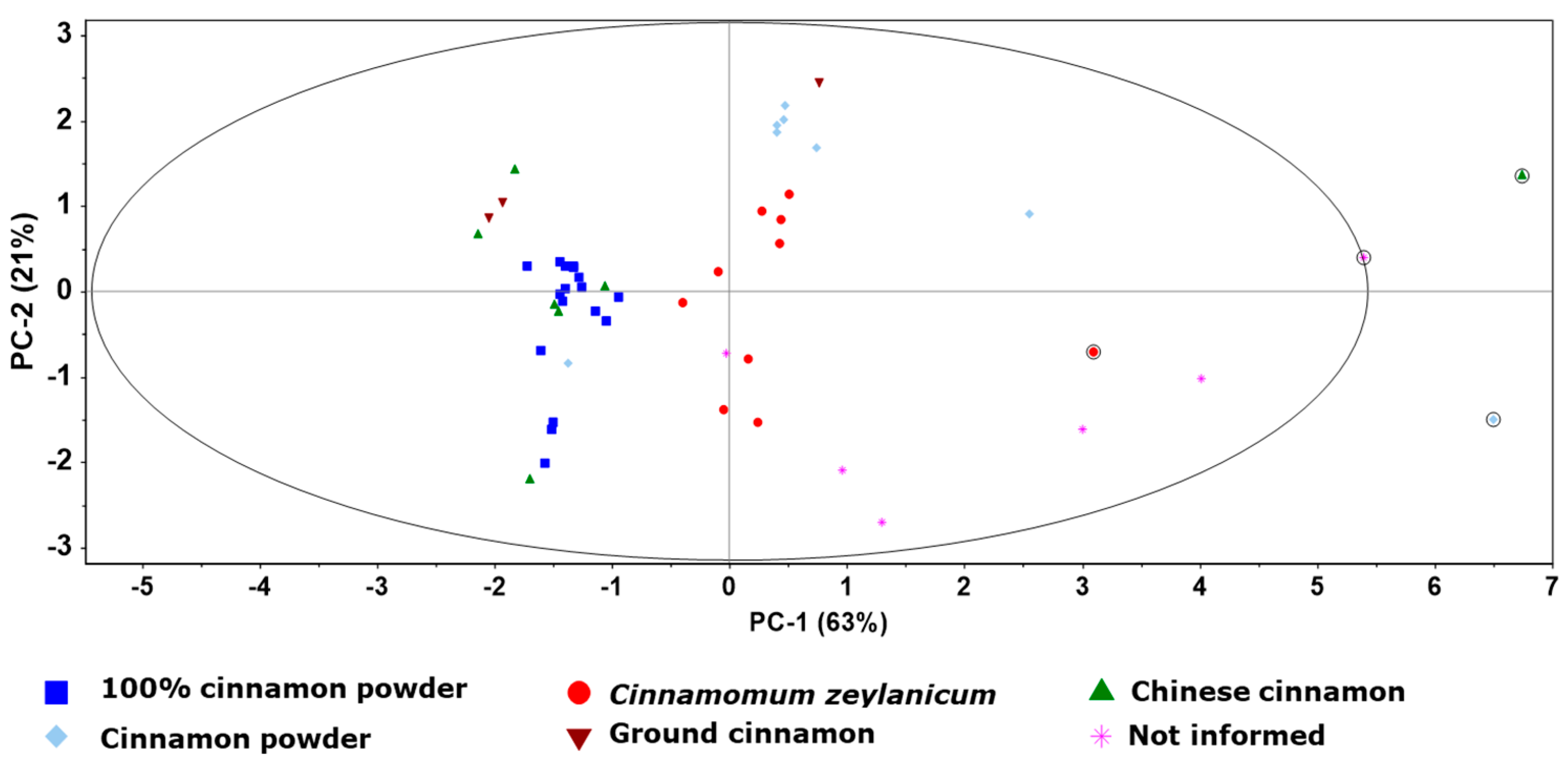
3.2. Mineral Composition and Sample Classification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Alternat. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Safdar, M. Proximate composition and mineral analysis of cinnamon. Pak. J. Nutr. 2009, 8, 1456–1460. [Google Scholar] [CrossRef]
- Qin, B.; Panickar, K.S.; Anderson, R.A. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J. Diabetes Sci. Technol. 2010, 4, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Silvis, I.C.J.; van Ruth, S.M.; van der Fels-Klerx, H.J.; Luning, P.A. Assessment of food fraud vulnerability in the spices chain: An explorative study. Food Control 2017, 81, 80–87. [Google Scholar] [CrossRef]
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/cinnamon-market (accessed on 16 October 2020).
- Jeremić, K.; Kladar, N.; Vučinić, N.; Todorović, N.; Hitl, M.; Lalić-Popović, M.; Gavarić, N. Morphological characterization of cinnamon bark and powder available in the Serbian market. Biol. Serbica 2019, 41, 89–39. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, P.; Downey, G. Multivariate class modeling for the verification of food-authenticity claims. Trends Anal. Chem. 2012, 35, 74–86. [Google Scholar] [CrossRef]
- Ye, X.; Jin, S.; Wang, D.; Zhao, F.; Yu, Y.; Zheng, D.; Ye, N. Identification of the origin of white tea based on mineral element content. Food Anal. Methods 2017, 10, 191–199. [Google Scholar] [CrossRef]
- Beltrán, M.; Sánchez-Astudillo, M.; Aparicio, R.; García-González, D.L. Geographical traceability of virgin olive oils from south-western Spain by their multi-elemental composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, M.C.A.; Martins, C.A.; Pozebon, D.; Dressler, V.L.; Ferrão, M.F. Classification of yerba mate (Ilex paraguariensis) according to the country of origin based on element concentrations. Microchem. J. 2014, 117, 164–171. [Google Scholar] [CrossRef]
- Kokhar, S.; Garduño-Diaz, S.D.; Marletta, L.; Shahar, D.R.; Ireland, J.D.; Jansen-van der Vliet, M.; Henauw, S. Mineral composition of commonly consumed ethnic foods in Europe. Food Nutr. Res. 2012, 56, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chudzinska, M.; Baralkiewicz, D. Estimation of honey authenticity by multielements characteristics using inductively coupled plasma-mass spectrometry (ICP-MS) combined with chemometrics. Food Chem. Toxicol. 2010, 48, 284–290. [Google Scholar] [CrossRef]
- Barbosa, R.M.; Batista, B.L.; Barião, C.V.; Varrique, R.M.; Coelho, V.A.; Campiglia, A.D.; Barbosa, F., Jr. A simple and practical control of the authenticity of organic sugarcane samples based on the use of machine-learning algorithms and trace elements determination by inductively coupled plasma mass spectrometry. Food Chem. 2015, 184, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.G.A.; Boa Morte, E.S.; Santos, D.C.M.B.; Castro, J.T.; Barbosa, J.T.P.; Teixeira, A.P.; Fernandes, A.P.; Welz, B.; Santos, W.P.C.; Santos, E.B.G.N.; et al. Sample Preparation for the Determination of Metals in Food Samples Using Spectroanalytical Methods—A Review. Appl. Spectrosc. Rev. 2008, 43, 67–92. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Ahmed, S.E.B.; Ahmad, D.; Al-Assaf, A.H. Nutritive value, levels of polyphenols and anti-nutritional factors in Sri Lankan cinnamon (Cinnamomum zeyalnicum) and Chinese cinnamon (cinnamomum cassia). Res. Bult. Food Sci. Agric. Res. Cent. 2007, 154, 5–21. [Google Scholar]
- United States Department of Agriculture. Available online: https://www.nal.usda.gov/fnic/food-composition (accessed on 16 October 2020).
| Element (Spectral Line) | Minimum Found | Maximum Found | NIST 1515 Found | NIST 1515 Certified Values | LOD | LOQ |
|---|---|---|---|---|---|---|
| P (213.618 nm) | 5.19 ± 0.08 | 1223 ± 22 | 1583 ± 2 | 1610 ± 20 | 0.03 | 0.08 |
| S (182.034 nm) | 7.59 ± 0.06 | 1681 ± 16 | 1078 ± 6 | N.A. | 0.05 | 0.14 |
| Mg (280.270 nm) | 479 ± 2 | 1352 ± 17 | 2835 ± 8 | 2710 ± 80 | 0.06 | 0.18 |
| Ca (422.673 nm) | 5300 ± 30 | 13225 ± 49 | 13720 ± 93 | 15,260 ± 150 | 0.34 | 1.01 |
| K (766.490 nm) | 3877 ± 27 | 7183 ± 74 | 14,075 ± 175 | 16,100 ± 100 | 0.32 | 0.95 |
| Cu (324.754 nm) | 2.55 ± 0.03 | 10.5 ± 0.1 | 5.50 ± 0.07 | 5.6 ± 0.2 | 0.04 | 0.12 |
| Zn (213.856 nm) | 5.4 ± 0.1 | 24 ± 1 | 12 ± 1 | 12.5 ± 0.3 | 0.04 | 0.11 |
| B (249.773 nm) | 9.34 ± 0.04 | 17 ± 1 | 31.50 ± 0.06 | 27 ± 2 | 0.02 | 0.06 |
| Fe (259.940 nm) | 18.0 ± 0.5 | 1994 ± 49 | 61.6 ± 0.6 | 83 ± 5 | 0.01 | 0.04 |
| Al (396.152 nm) | 28.4 ± 0.4 | 2142 ± 13 | 295.87 ± 16.91 | 286 ± 9 | 0.10 | 0.30 |
| Mn (257.610 nm) | 137.9 ± 0.3 | 367 ± 2 | 56.11 ± 0.03 | 54 ± 3 | 0.0002 | 0.0006 |
| Si (251.611 nm) | 40.4 ± 0.9 | 2743 ± 52 | 551.24 ± 3.48 | N.A. | 0.60 | 1.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.F.S.; Martins, L.C.; Moraes, L.M.B.; Gonçalves, I.C.; de Godoy, B.B.R.; Erasmus, S.W.; van Ruth, S.; Rocha, F.R.P. Can Minerals Be Used as a Tool to Classify Cinnamon Samples? Proceedings 2021, 70, 22. https://doi.org/10.3390/foods_2020-07652
Silva AFS, Martins LC, Moraes LMB, Gonçalves IC, de Godoy BBR, Erasmus SW, van Ruth S, Rocha FRP. Can Minerals Be Used as a Tool to Classify Cinnamon Samples? Proceedings. 2021; 70(1):22. https://doi.org/10.3390/foods_2020-07652
Chicago/Turabian StyleSilva, Anna Flavia S., Luís Cláudio Martins, Liz M. B. Moraes, Isabela C. Gonçalves, Bianca B. R. de Godoy, Sara W. Erasmus, Saskia van Ruth, and Fábio R. P. Rocha. 2021. "Can Minerals Be Used as a Tool to Classify Cinnamon Samples?" Proceedings 70, no. 1: 22. https://doi.org/10.3390/foods_2020-07652
APA StyleSilva, A. F. S., Martins, L. C., Moraes, L. M. B., Gonçalves, I. C., de Godoy, B. B. R., Erasmus, S. W., van Ruth, S., & Rocha, F. R. P. (2021). Can Minerals Be Used as a Tool to Classify Cinnamon Samples? Proceedings, 70(1), 22. https://doi.org/10.3390/foods_2020-07652






