Abstract
This work focuses on a capacitive biosensor based on a hydride peptide for the detection of MMP-13. Indeed, the enzyme MMP-13 is a remarkable indicator of inflammation in chronic wounds. To achieve specific detection of this enzyme, a metallocene was added to the peptide which amplifies the electrical variation allowing for proof of concept and reliability.
1. Introduction
Many enzymes are involved in the healing of chronic wounds, and some are also involved in bacterial colonization [1]. MMP-13 is one such enzymes, and is remarkable in that it is a sign of inflammation in this type of wound. Indeed, unlike other MMPs that play an important physiological role in wound healing processes, the presence of MMP-13 is therefore synonymous with poor wound healing [2]. In the present work, we developed a capacitive biosensor functionalized with a hybrid peptide that degrades only in the presence of the target MMP. This biosensor is based on an interdigitated capacitor functionalized by a peptide substrate modified with the addition of a metallocene such as ferrocene carboxylic acid. This addition is part of the piece of the peptide sequence cut by the target enzyme allowing the release from the active surface of the biosensor and thus leading to a modification of the electrical response. To achieve this goal, several hybrid peptides were designed and evaluated through structural and electrical characterization. To conclude, the sensitivity and specificity to MMP13 were verified.
2. Materials and Methods
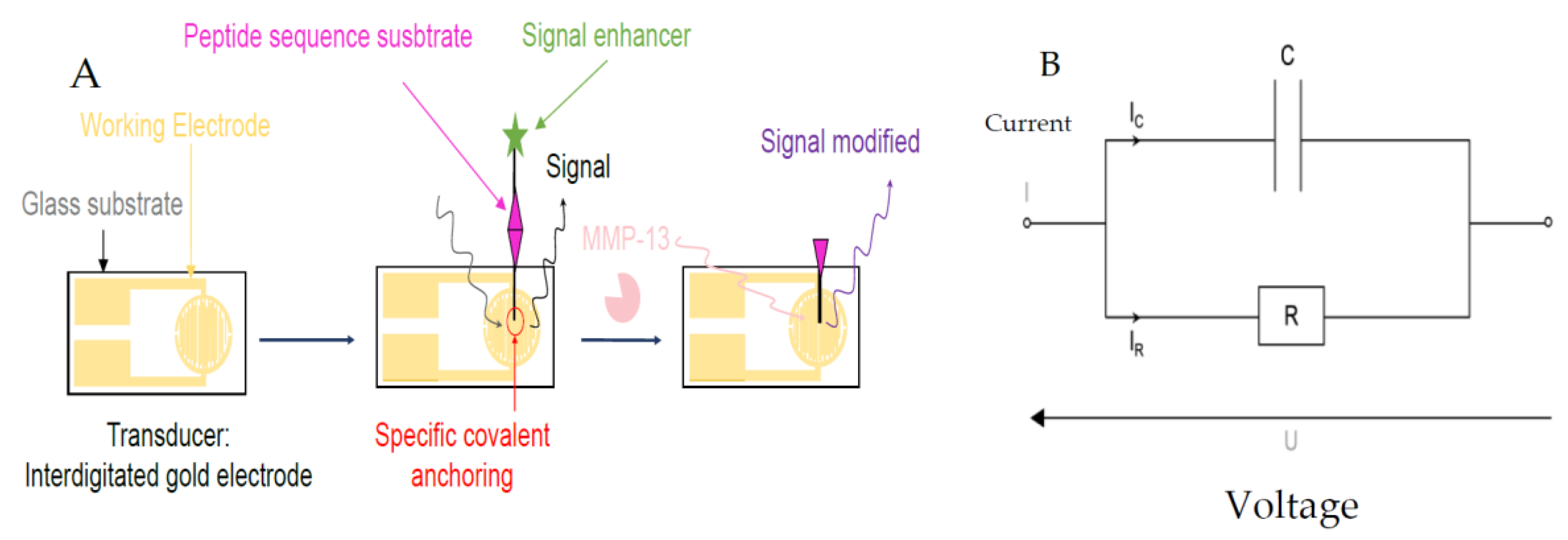
Figure 1 shows the biosensor principle, and the methodology used to achieve the objective. We used a commercial gold interdigital electrode (IDE) (10 mm × 6 mm) from Micrux Company [3] with an active area of around 10 mm2. Before immobilization of hybrid peptides on the IDE, we need a trialkoxysilanes layer to fix it onto the IDE gold surface. This is obtained with 3-mercaptopropyl trimethoxysilane [4] (MPTMS) with 1% v/v. Then, the hybrid peptide solution is deposited by drop casting (3 µL–10 mM of EtOH/H2O-HCl v/v 98/2 solution at pH 1.4). The theoretical density of hybrid peptides grafted is 301 peptides/nm2. Indeed, the actual grafting of the hybrid peptide layer and its resistance to washings was confirmed by X-ray photoelectron spectroscopy (XPS).
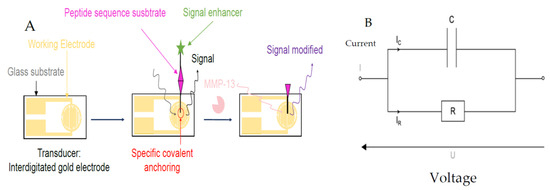
Figure 1.
(A) Peptide-based sensor for MMP-13 activity detection. (B) Electrical model used for measurement.
Then, the electrical measurements were performed under a specific probe in a climatic chamber using an LF Impedance Analyzer 5 Hz–10 MHz (4192 A HP) controlled by a Labview software 21.0. Samples were analyzed with a frequency range of 100 Hz to 100 KHz and an oscillation amplitude of 250 mV.
3. Results and Discussion
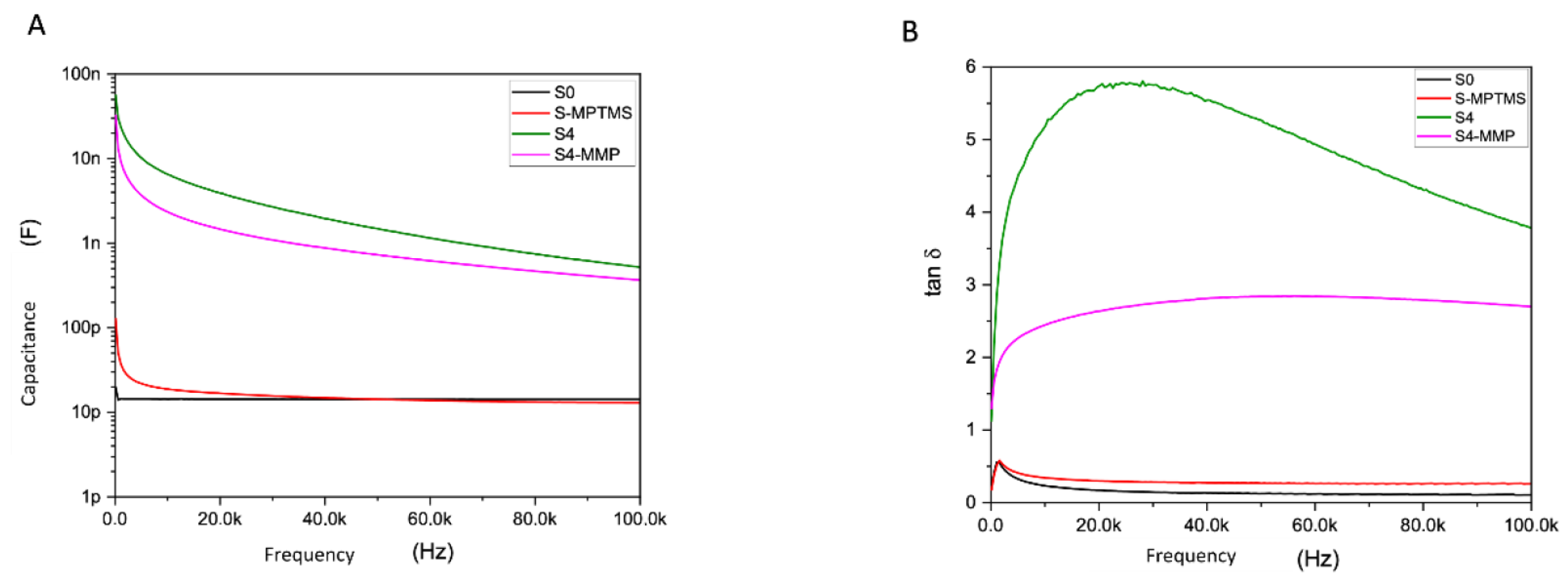
The modelling of our biosensor is a parallel R-C circuit type electrical model. The electrical parameters monitored throughout the experiments are the capacitance (C) and the dissipation factor (tan δ), one of which reflects the electrical charge (C) and the other the losses due to a non-negligible loss current across the resistive part (R) (see Figure 1). Through all the electrical measurements, we demonstrate that our hybrid peptide is well functionalized immobilized. It is resistant to washes; moreover, the addition of the ferrocene group allows us to obtain a higher sensitivity, and it reacts only to MMP-13. Figure 2A,B shows the electrical response with C and tan δ from 100 Hz to 100 KHz, with the black curve (S0) representing the IDE. The red curve (S-MPTMS) contains just the precursor layer, the green (S4) is totally grafted with hybrid peptide, and the pink is the same as S4 but after the MMP-13 treatment. As we can see, when the S4 sample was in contact with a solution containing the target enzyme, the MMP enzyme activity resulted in a deterioration of the peptide and a measurable electrical change (Figure 2A,B).
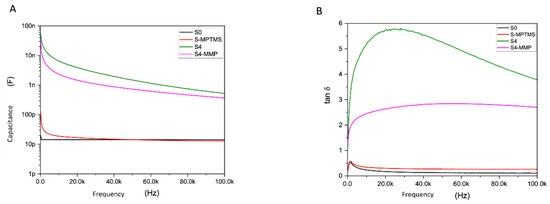
Figure 2.
Curves representing the degradation of the S4 material (green curve) by MMP-13 (S4-MMP, pink curve). (A) capacitance versus frequency. (B) Tan δ versus frequency.
Author Contributions
Sol-gel approach, A.M.; Hybrid peptide design and synthesis, C.E.; Conceptualization, A.M., B.S. and G.S.; methodology, A.M., B.S. and G.S.; software, A.V.; Electrical measurement, validation, A.S., A.V. and B.S.; formal analysis, A.S., A.M., B.S. and G.S.; writing—review and editing, B.S.; supervision, G.S.; funding acquisition, B.S and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MUSE (Montpellier Université excellence) AAP2017 “e-Dressing project”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch. Dermatol. 1994, 130, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Westby, M.J.; Dumville, J.C.; Stubbs, N.; Norman, G.; Wong, J.K.; Cullum, N.; Riley, R.D. Dresssings and topical agents for treating pressure ulcers. Cochrane Database Syst. Rev. 2018, CD011947. [Google Scholar] [CrossRef]
- Available online: https://www.micruxfluidic.com/en/ (accessed on 31 March 2023).
- Masurier, N.; Tissot, J.-B.; Boukhriss, D.; Jebors, S.; Pinese, C.; Verdie, P.; Mehdi, A.; Martinez, J.; Humblot, V.; Subra, G. Site-specific grafting on titanium surfaces with hybrid temporin antibacterial peptides. J. Mater. Chem. B 2018, 6, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).