Studies of Effects of Calcination Temperature on the Crystallinity and Optical Properties of Ag-Doped ZnO Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Ag/ZnO
2.3. Characterization of Ag/ZnO
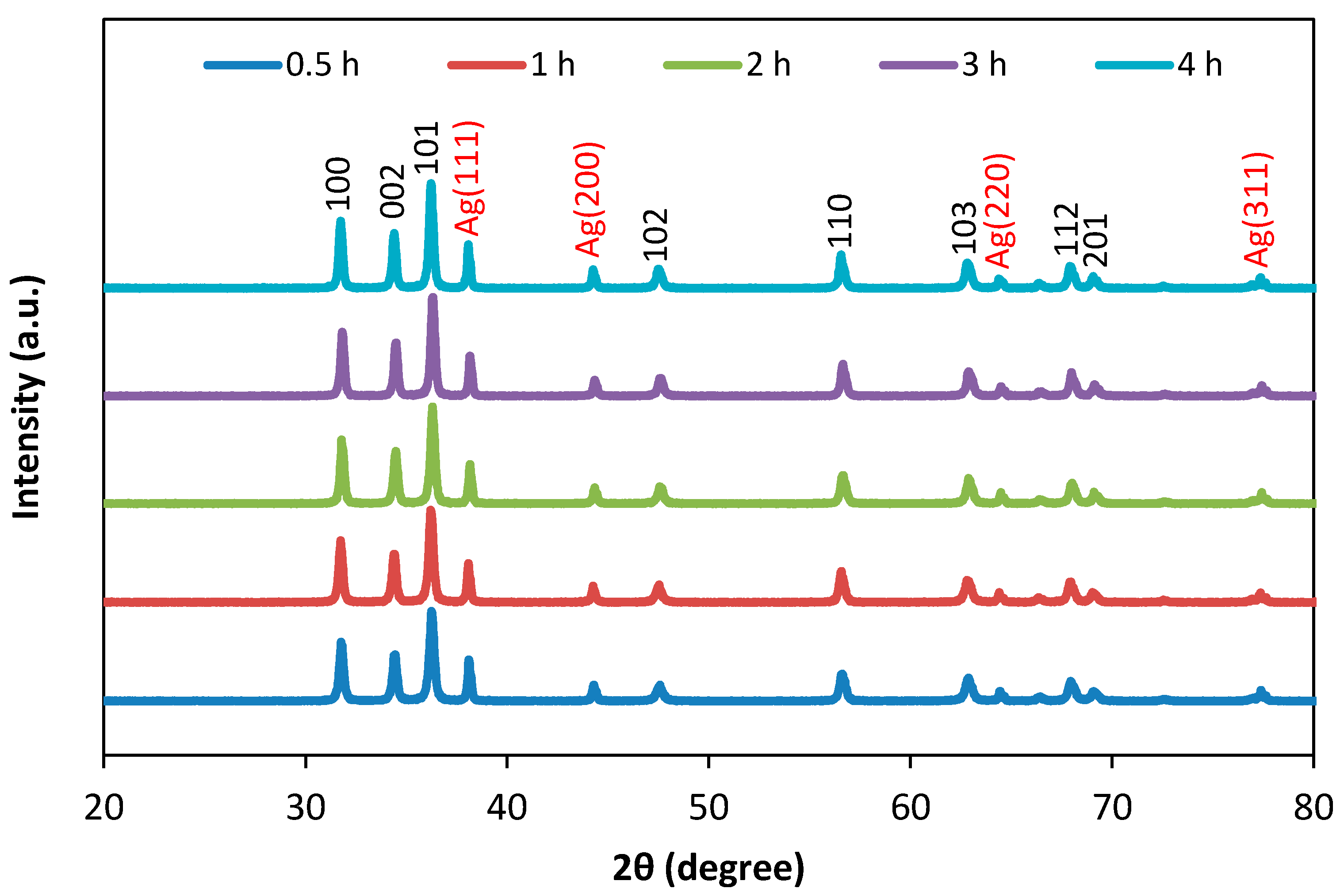
3. Results and Discussion
3.1. Structural Analysis
3.2. Morphology study
3.3. Diffuse Reflectance Spectroscopic (DRS) Spectra
3.4. Photoluminescence Study
3.5. X-Ray Photoelectron Spectroscopic (XPS) Spectra
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sánchez Zeferino, R.; Barboza Flores, M.; Pal, U. Photoluminescence and raman scattering in Ag-doped ZnO nanoparticles. J. Appl. Phys. 2011, 109, 014308. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Cho, M.H. Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J. Chem. 2017, 41, 9314–9320. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-based ultraviolet photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Djaja, N.F. Transition-metal-doped ZnO nanoparticles: Synthesis, characterization and photocatalytic activity under UV light. Spectrochim. Acta A 2014, 130, 581–590. [Google Scholar] [CrossRef]
- Habibi, M.H.; Rahmati, M.H. The effect of operational parameters on the photocatalytic degradation of Congo red organic dye using ZnO—CdS core–shell nano-structure coated on glass by Doctor Blade method. Spectrochim. Acta A 2015, 137, 160–164. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Ansari, S.A.; Parveen, N.; Cho, M.H.; Song, T. Lithium ion storage ability, supercapacitor electrode performance, and photocatalytic performance of tungsten disulfide nanosheets. New J. Chem. 2018, 42, 5859–5867. [Google Scholar] [CrossRef]
- Wang, D.; Song, C. Controllable Synthesis of ZnO Nanorod and Prism Arrays in a Large Area. J. Phys. Chem. B 2005, 109, 12697–12700. [Google Scholar] [CrossRef]
- Feng, W.H.; Wang, B.; Zheng, Z.Y.; Fang, Z.B.; Wang, Z.F.; Zhang, S.Y.; Li, Y.H.; Liu, P. Predictive model for optimizing the near–field electromagnetic energy transfer in plasmonic nanostructure–involved photocatalysts. Appl. Catal. B 2016, 186, 143–150. [Google Scholar] [CrossRef]
- Zhao, L.; Lian, J.; Liu, Y.; Jiang, Q. Structural and optical properties of ZnO thin films deposited on quartz glass by pulsed laser deposition. Appl. Surf. Sci. 2006, 252, 8451–8455. [Google Scholar] [CrossRef]
- Türkyılmaza, Ş.Ş.; Güya, N.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A Chem. 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Hou, X.M. ZnO/Ag heterostructured nanoassemblies: Wet–chemical preparation and improved visible–light photocatalytic performance. Mater. Lett. 2015, 139, 201–204. [Google Scholar] [CrossRef]
- Park, W.; Jone, T.C.; Summers, C.J. Optical properties of SrS:Cu,Ag two-component phosphors for electroluminescent devices. Appl. Phys. Lett. 1999, 74, 1785–1787. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Sun, W.; Huang, J.; Xie, H.; Zhao, X. Surface modification of ZnO with Ag improves its photocatalytic efficiency and photostability. J. Photochem. Photobiol. A 2010, 216, 149–155. [Google Scholar] [CrossRef]
- Lee, M.-K.; Kim, T.G.; Kim, W.; Sung, Y.-M. Surface plasmon resonance (SPR) electron and energy transfer in noble metal-zinc oxide composite nanocrystals. J. Phys. Chem. C 2008, 112, 10079–10082. [Google Scholar] [CrossRef]
- Dong, Y.M.; Feng, C.Y.; Jiang, P.P.; Wang, G.L.; Li, K.; Miao, H.Y. Simple one-pot synthesis of ZnO/Ag heterostructures and the application in visible-light-responsive photocatalysis. RSC Adv. 2014, 4, 7340–7346. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; He, H.; Wang, Q.; Xing, L.; Xue, X. Enhanced piezo/solar-photocatalytic activity of Ag/ZnO nanotetrapods arising from the coupling of surface plasmon resonance and piezophototronic effect. J. Phys. Chem. Solids 2017, 102, 27–33. [Google Scholar] [CrossRef]
- Xue, X.; Zang, W.; Deng, P.; Wang, Q.; Xing, L.; Zhang, Y.; Wang, Z.L. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Li, Z.; Song, J.; Wang, Z.L. Single-Crystal Mesoporous ZnO Thin Films Composed of Nanowalls. J. Phys. Chem. C 2009, 113, 1791–1794. [Google Scholar] [CrossRef] [Green Version]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A highly efficient Ag–ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, N.; Li, L.; Li, R.; Ji, G.; Gan, S. Fabrication of porous 3D flower–like Ag/ZnO heterostructure composites with enhanced photocatalytic performance. Appl. Surf. Sci. 2015, 332, 32–39. [Google Scholar] [CrossRef]
- Potti, P.R.; Srivastava, V.C. Comparative studies on structural, optical, and textural properties of combustion derived ZnO prepared using various fuels and their photocatalytic activity. Ind. Eng. Chem. Res. 2012, 51, 7948–7956. [Google Scholar] [CrossRef]
- Rokesh, K.; Mohan, S.C.; Karuppuchamy, S.; Jothivenkatachalam, K. Photo-assisted advanced oxidation processes for Rhodamine B degradation using ZnO–Ag nanocomposite materials. J. Environ. Chem. Eng. 2018, 6, 3610–3620. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Photocatalytic activity of Ag/ZnO heterostructure nanocatalyst: Correlation between structure and property. J. Phys. Chem. C 2008, 112, 10773–10777. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Growth of three-dimensional flower-like SnS2 on g-C3N4 sheets as an efficient visible-light photocatalyst, photoelectrode, and electrochemical supercapacitance material. Sustain. Energy Fuels 2017, 1, 510–519. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Pourahmad, A.; Salavatiyan, T. CuO–MMT nanocomposite: Effective photocatalyst for the discoloration of methylene blue in the absence of H2O2. Appl. Phys. A 2016, 122, 111–117. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue luminescence of ZnO nanoparticles based on non–equilibrium processes: Defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Rokesh, K.; Pandikumar, A.; Jothivenkatachalam, K. Zinc oxide nanopillar: Preparation, characterization and its photoelectrocatalytic activity. Mater. Focus 2014, 3, 345–349. [Google Scholar] [CrossRef]
- Zhang, D.H.; Wang, Q.P.; Xue, Z.Y. Photoluminescence of ZnO films excited with light of different wavelength. Appl. Surf. Sci. 2003, 207, 20–25. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, L.; Wang, D.; Lin, Y.; He, D.; Xie, T. UV–illumination room–temperature gas sensing activity of carbon–doped ZnO microspheres. Sens. Actuators B Chem. 2012, 161, 292–297. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Lee, J.; Cho, M.H. Biogenic synthesis, photocatalytic, and photoelectrochemical performance of Ag–ZnO nanocomposite. J. Phys. Chem. C 2013, 117, 27023–27030. [Google Scholar] [CrossRef]
- Bai, H.W.; Liu, Z.Y.; Sun, D.D. Hierarchical ZnO/Cu corn–like materials with high photodegradation and antibacterial capability under visible light. Phys. Chem. Chem. Phys. 2011, 13, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Pradhan, S.; Sahu, D.; Mishra, D.; Sarangi, S.; Nayak, B.; Verma, S.; Roul, B. Micro–Raman and XPS studies of pure ZnO ceramics. Phys. B Condens. Matter 2010, 405, 2492–2497. [Google Scholar] [CrossRef]
- Khosravi–Gandomani, S.; Yousefi, R.; Jamali–Sheini, F.; Huang, N.M. Optical and electrical properties of p–type Ag–doped ZnO nanostructures. Ceram. Int. 2014, 40, 7957–7963. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Tamboli, M.S.; Patil, D.R.; Kulkarni, M.V.; Yoon, H.; Kim, H.; Al–Deyab, S.S.; Yoon, S.S.; Kolekar, S.S.; et al. Green approach for hierarchical nanostructured Ag–ZnO and their photocatalytic performance under sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Studies of Effects of Calcination Temperature on the Crystallinity and Optical Properties of Ag-Doped ZnO Nanocomposites. J. Compos. Sci. 2019, 3, 18. https://doi.org/10.3390/jcs3010018
Molla MAI, Furukawa M, Tateishi I, Katsumata H, Kaneco S. Studies of Effects of Calcination Temperature on the Crystallinity and Optical Properties of Ag-Doped ZnO Nanocomposites. Journal of Composites Science. 2019; 3(1):18. https://doi.org/10.3390/jcs3010018
Chicago/Turabian StyleMolla, Md. Ashraful Islam, Mai Furukawa, Ikki Tateishi, Hideyuki Katsumata, and Satoshi Kaneco. 2019. "Studies of Effects of Calcination Temperature on the Crystallinity and Optical Properties of Ag-Doped ZnO Nanocomposites" Journal of Composites Science 3, no. 1: 18. https://doi.org/10.3390/jcs3010018
APA StyleMolla, M. A. I., Furukawa, M., Tateishi, I., Katsumata, H., & Kaneco, S. (2019). Studies of Effects of Calcination Temperature on the Crystallinity and Optical Properties of Ag-Doped ZnO Nanocomposites. Journal of Composites Science, 3(1), 18. https://doi.org/10.3390/jcs3010018




