Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of CNC Suspension
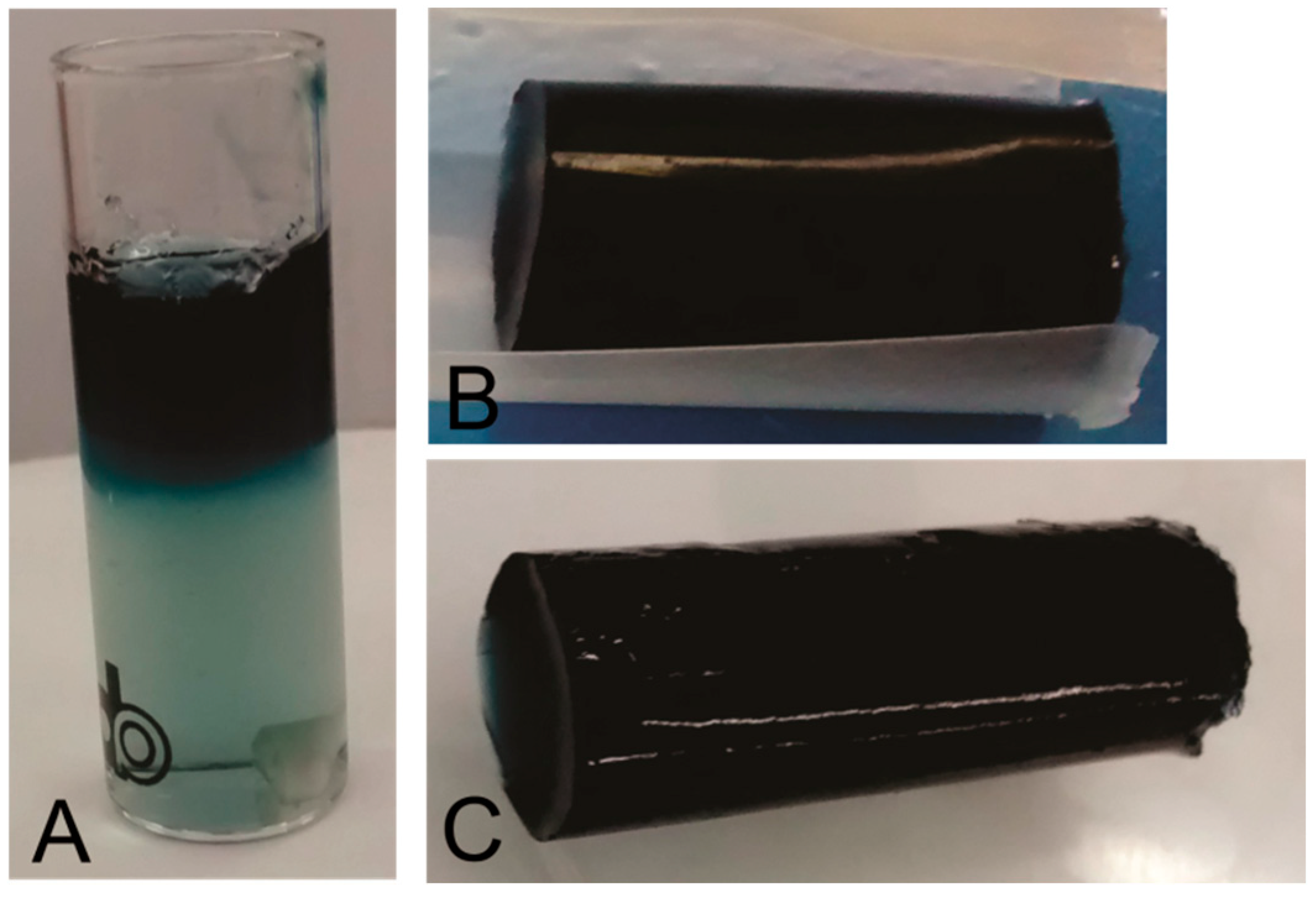
3.2. Chitosan Crosslinking by Genipin
3.3. Porosity
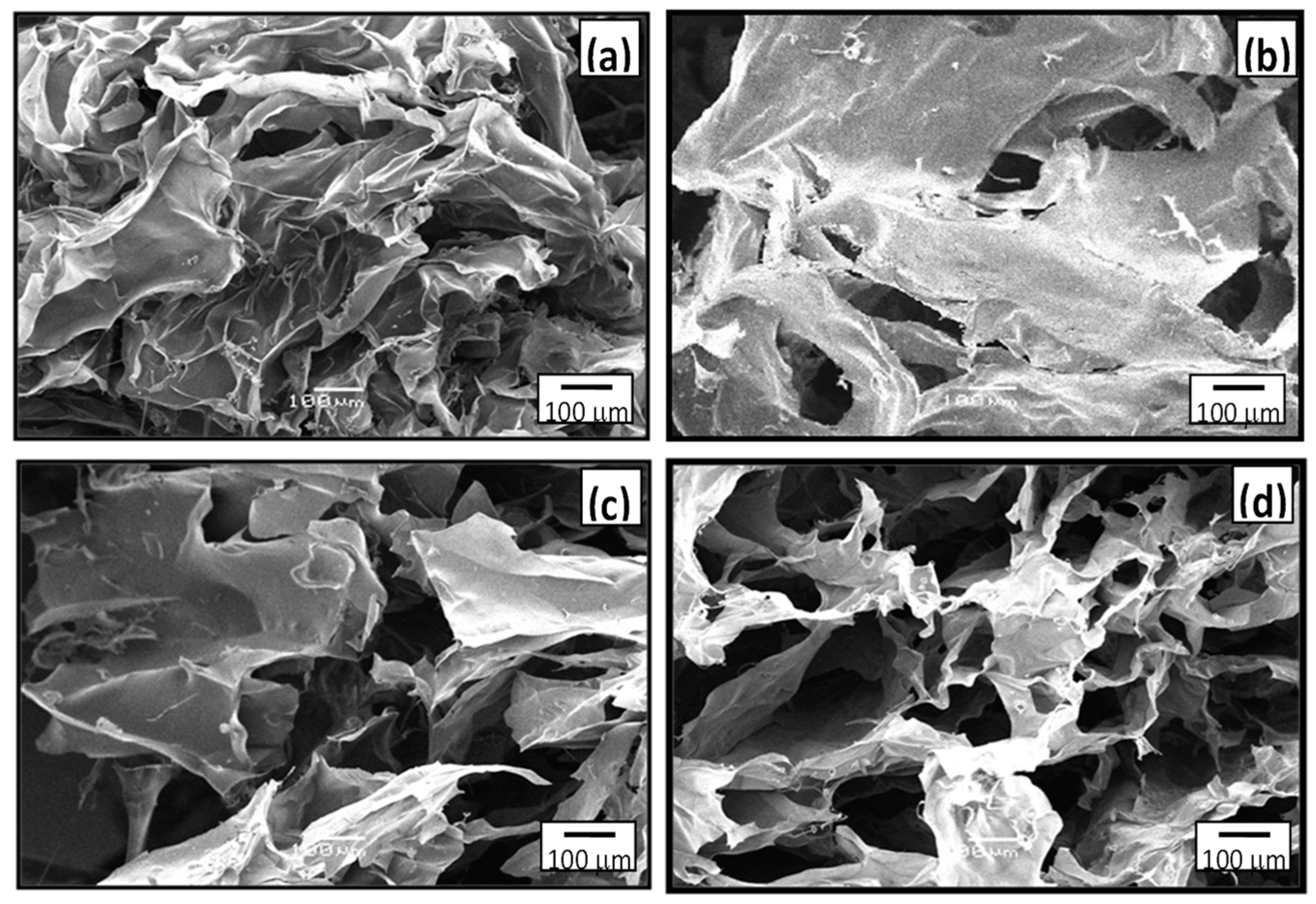
3.4. Scanning Electron Microscopy
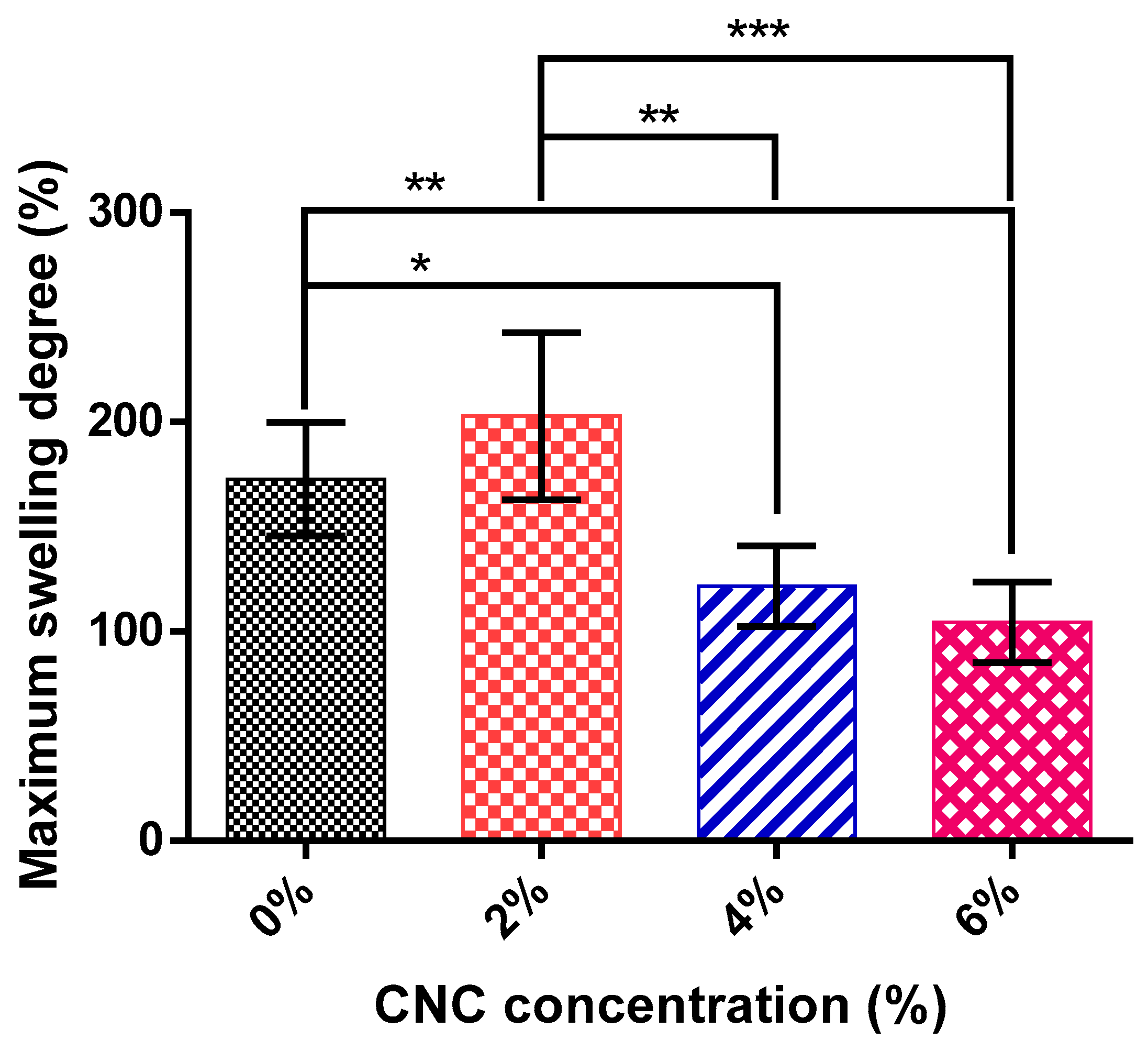
3.5. Swelling Tests
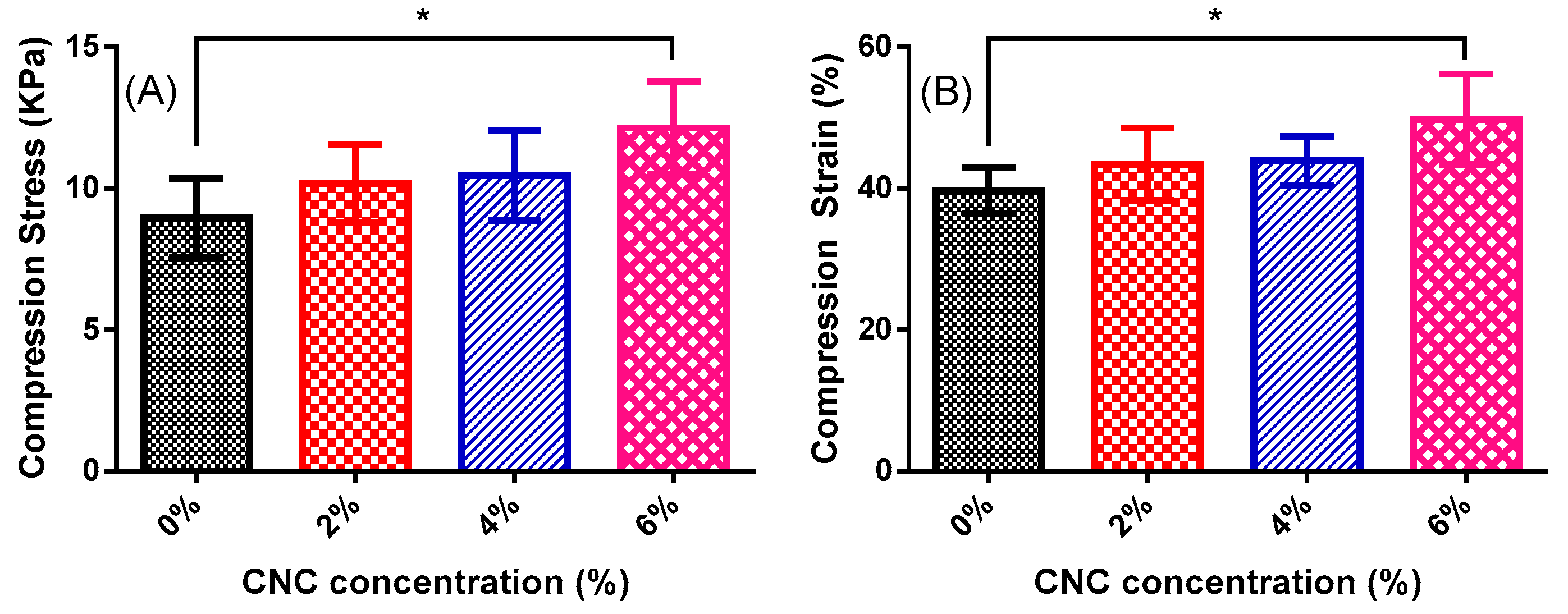
3.6. Compression Mechanical Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montanheiro, T.L.A.; Montagna, L.S.; Patrulea, V.; Jordan, O.; Borchard, G.; Lobato, G.M.M.; Catalani, L.H.; Lemes, A.P. Evaluation of cellulose nanocrystal addition on morphology, compression modulus and cytotoxicity of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds. J. Mater. Sci. 2019, 54, 7198–7210. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, R.; Cheng, Y.; Fu, J. Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter 2012, 8, 6048–6056. [Google Scholar] [CrossRef]
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Meißner, M.; Schlie-Wolter, S.; Chichkov, B.N. Water-soluble photopolymerizable chitosan hydrogels for biofabrication via two-photon polymerization. Acta Biomater. 2015, 18, 186–195. [Google Scholar] [CrossRef] [PubMed]
- De Mesquita, J.P.; Donnici, C.L.; Teixeira, I.F.; Pereira, F.V. Bio-based nanocomposites obtained through covalent linkage between chitosan and cellulose nanocrystals. Carbohydr. Polym. 2012, 90, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Vimala, K.; Mohan, Y.M.; Sivudu, K.S.; Varaprasad, K.; Ravindra, S.; Reddy, N.N.; Padma, Y.; Sreedhar, B.; MohanaRaju, K. Fabrication of porous chitosan films impregnated with silver nanoparticles: A facile approach for superior antibacterial application. Colloids Surf. B 2010, 76, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.H.; Camitz, L.; Illigen, K.E.; Hansen, M.; Sarvaa, R.; Conaghan, P.G. The effects of polyacrylamide hydrogel in normal and osteoarthritic animal joints. Osteoarthr. Cartilage 2016, 24, S449–S450. [Google Scholar] [CrossRef]
- Ferreira, S.D.O.; Montanheiro, T.L.A.; Ontagna, L.S.; Lemes, A.P. Study of cellulose nanocrystals and zinc nitrate hexahydrate addition in chitosan. Mater. Res. 2019, 22, 1–10. [Google Scholar] [CrossRef]
- Ke, Y.; Wu, G.; Wang, Y. PHBV/PAM scaffolds with local oriented structure through UV polymerization for tissue engineering. Biomed Res. Int. 2014, 2014, 157987. [Google Scholar] [CrossRef]
- Hong, Y.; Song, H.; Gong, Y.; Mao, Z.; Gao, C.; Shen, J. Covalently crosslinked chitosan hydrogel: Properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 2007, 3, 23–31. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Kim, H. pH-responsive release behavior of genipin-crosslinked chitosan/poly(ethylene glycol) hydrogels. J. Appl. Polym. Sci. 2012, 125, 36899. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Gonsalves, A.A.; Araújo, C.R.M.; Soares, N.A.; Goulart, M.O.F.; Abreu, F.C. de Diferentes estratégias para a reticulação de quitosana. Quim. Nova 2011, 34, 1215–1223. [Google Scholar] [CrossRef]
- Hortigüela, M.J.; Aranaz, I.; Gutiérrez, M.C.; Ferrer, M.L.; Del Monte, F. Chitosan gelation induced by the in situ formation of gold nanoparticles and its processing into macroporous scaffolds. Biomacromolecules 2011, 12, 179–186. [Google Scholar] [CrossRef]
- Montanheiro, T.L.A.; Montagna, L.S.; de Farias, M.A.; Lemes, A.P. Cytotoxicity and physico-chemical evaluation of acetylated and pegylated cellulose nanocrystals. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Zhang, L. Structure and properties of the nanocomposite films of chitosan reinforced with cellulose whiskers. J. Polym. Sci. Part B Polym. Phys. 2003, 47, 1069–1077. [Google Scholar] [CrossRef]
- Geng, C.Z.; Hu, X.; Yang, G.; Zhang, Q.; Chen, F.; Fu, Q. Mechanically reinforced chitosan/cellulose nanocrystals composites with good transparency and biocompatibility. Chinese J. Polym. Sci. 2015, 33, 61–69. [Google Scholar] [CrossRef]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Tien, C.L.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Algan, C. Electrospinning of Chitosan-Based Nanocomposites Reinforced with Biobased Nanocrystals for Biomedical Applications. Master’s Thesis, Luleå University of Technology, Lulea, Sweden, October 2013. [Google Scholar]
- Sultana, N.; Wang, M. PHBV tissue engineering scaffolds fabricated via emulsion freezing / freeze-drying: effects of processing parameters. Int. Conf. Biomed. Eng. Technol. 2011, 11, 29–34. [Google Scholar]
- Sankar, D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Fabrication of chitin/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) hydrogel scaffold. Carbohydr. Polym. 2012, 90, 725–729. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Emam, H.E. Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using Acid hydrolysis. Int. J. Biol. Macromol. 2018, 107, 1599–1606. [Google Scholar] [CrossRef]
- Song, K.; Ji, Y.; Wang, L.; Wei, Y.; Yu, Z. A green and environmental benign method to extract cellulose nanocrystal by ball mill assisted solid acid hydrolysis. J. Clean. Prod. 2018, 196, 1169–1175. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, S.; Ma, Y. International journal of biological macromolecules isolation and characterization of cellulose nanocrystals from pueraria root residue. Int. J. Biol. Macromol. 2019, 129, 1081–1089. [Google Scholar] [CrossRef]
- Mutjé, P.; Lòpez, A.; Vallejos, M.E.; López, J.; Vilaseca, F. Full exploitation of Cannabis sativa as reinforcement / filler of thermoplastic composite materials. Composites Part A 2007, 38, 369–377. [Google Scholar] [CrossRef]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef]
- Pujana, M.A.; Pérez-álvarez, L.; Carlos, L.; Iturbe, C.; Katime, I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydr. Polym. 2013, 94, 836–842. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part. A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef]
- Wang, S.; Sun, C.; Guan, S.; Li, W.; Xu, J.; Ge, D.; Zhuang, M.; Liu, T.; Ma, X. Chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B 2017, 5, 4774–4788. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Mansur, H.S. Engineered 3D-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater. Sci. Eng. C 2017, 78, 690–705. [Google Scholar] [CrossRef]
- Naseri, N.; Poirier, J.M.; Girandon, L.; Fröhlich, M.; Oksman, K.; Mathew, A.P. 3-Dimensional porous nanocomposite scaffolds based on cellulose nanofibers for cartilage tissue engineering: Tailoring of porosity and mechanical performance. RSC Adv. 2016, 6, 5999–6007. [Google Scholar] [CrossRef]
- Wu, T.; Farnood, R.; Kelly, K.O.; Chen, B. Mechanical behavior of transparent nano fibrillar cellulose–chitosan nanocomposite films in dry and wet conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 279–286. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Dufresne, A.; Pinheiro, I.F.; Souza, D.H.S.; Gouveia, R.F.; Mei, L.H.I.; Lona, L.M.F. How do cellulose nanocrystals affect the overall properties of biodegradable polymer nanocomposites: A comprehensive review. Eur. Polym. J. 2018, 108, 274–285. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, J.; Feng, X.; Zhang, H.; Guo, Y.; Chen, J. Fabrication and characterization of bioactive silk fibroin/wollastonite composite scaffolds. Mater. Sci. Eng. C 2010, 30, 132–140. [Google Scholar] [CrossRef]
- Sabree, I.; Gough, J.E.; Derby, B. Mechanical properties of porous ceramic scaffolds: Influence of internal dimensions. Ceram. Int. 2015, 41, 8425–8432. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomari, A.A.d.N.; Montanheiro, T.L.d.A.; de Siqueira, C.P.; Silva, R.S.; Tada, D.B.; Lemes, A.P. Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization. J. Compos. Sci. 2019, 3, 84. https://doi.org/10.3390/jcs3030084
Pomari AAdN, Montanheiro TLdA, de Siqueira CP, Silva RS, Tada DB, Lemes AP. Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization. Journal of Composites Science. 2019; 3(3):84. https://doi.org/10.3390/jcs3030084
Chicago/Turabian StylePomari, Andréia Aparecida do Nascimento, Thaís Larissa do Amaral Montanheiro, Cristiane Pereira de Siqueira, Rodrigo Sousa Silva, Dayane Batista Tada, and Ana Paula Lemes. 2019. "Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization" Journal of Composites Science 3, no. 3: 84. https://doi.org/10.3390/jcs3030084
APA StylePomari, A. A. d. N., Montanheiro, T. L. d. A., de Siqueira, C. P., Silva, R. S., Tada, D. B., & Lemes, A. P. (2019). Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization. Journal of Composites Science, 3(3), 84. https://doi.org/10.3390/jcs3030084




