Evaluation of Freeze Drying and Electrospinning Techniques for Saffron Encapsulation and Storage Stability of Encapsulated Bioactives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Saffron Extracts
2.3. Preparation of Polymer Solution
2.4. Rheological Properties of the Polymer Solution
2.5. Encapsulation Process
2.5.1. Electrospinning
2.5.2. Freeze Drying Procedure
2.6. Characterization of Electro-Spun Fibers and Freeze-Dried Powders
2.6.1. Scanning Electron Microscopy
2.6.2. Determination of Encapsulation Efficiency
2.6.3. Stability of Encapsulated Saffron under Accelerated Storage Condition
2.6.4. Thermal Properties
2.6.5. Kinetics of Degradation of Crocin in Saffron Extract
2.7. Statistical Analysis
3. Results and Discussions
3.1. Apparent Viscosity of Polymer Solutions
3.2. Scanning Electron Microscopy (SEM)
3.3. Encapsulation Efficiency (EE)
3.4. Thermal Analysis
3.5. Storage Stability of Encapsulated Saffron Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández, J.A. Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant Sci. 2004, 2, 127–159. [Google Scholar]
- Basker, D.; Negbi, M. Uses of saffron. Econ. Bot. 1983, 37, 228–236. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Ahmed, M.; Akter, M.S.; Lee, J.C.; Eun, J.B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT-Food Sci. Technol. 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Dehcheshmeh, M.A.; Fathi, M. Production of core-shell nanofibers from zein and tragacanth for encapsulation of saffron extract. Int. J. Biol. Macromol. 2019, 122, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Uyar, T. Encapsulation of vanillin/cyclodextrin inclusion complex in Electro-spun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Garavand, F.; Rahaee, S.; Vahedikia, N.; Jafari, S.M. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends Food Sci. Technol. 2019, 89, 26–44. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Sharayei, P.; Zomorodi, S.; Ramaswamy, H.S. Physicochemical and Phytochemical Characterization and Storage Stability of Freeze-dried Encapsulated Pomegranate Peel Anthocyanin and In Vitro Evaluation of Its Antioxidant Activity. Food Bioprocess Technol. 2019, 12, 199–210. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Murali, S.; Kar, A.; Mohapatra, D.; Kalia, P. Encapsulation of black carrot juice using spray and Freeze drying. Food Sci. Technol. Int. 2015, 21, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, W.; Akay, H.; Pikal, M. A computational model for finite element analysis of the freeze drying process. Comput. Methods Appl. Mech. Eng. 1997, 148, 105–124. [Google Scholar] [CrossRef]
- Ceballos, A.M.; Giraldo, G.I.; Orrego, C.E. Effect of freezing rate on quality parameters of freeze-dried soursop fruit pulp. J. Food Eng. 2012, 111, 360–365. [Google Scholar] [CrossRef]
- Buffo, R.A.; Reineccius, G.A. Shelf-life and mechanisms of destabilization in dilute beverage emulsions. Flavour Fragr. J. 2001, 16, 7–12. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Shimoni, E. Overview of microencapsulates for use in food products or processes and methods to make them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–29. [Google Scholar]
- Ahmadian, Z.; Niazmand, R.; Pourfarzad, A. Microencapsulation of saffron petal phenolic extract: Their characterization, in vitro gastrointestinal digestion, and storage Stability. J. Food Sci. 2019, 84, 2745–2757. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.-Q.; Chen, Z.; Tao, Y.; Liu, Y.-F. Recent progress on thin-film encapsulation technologies for organic electronic devices. Opt. Commun. 2016, 362, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Liu, Z.; Tian, F.; Chen, S.; Lei, L.; Jiang, T.; Fan, Y. Sustained delivery of calcium and orthophosphate ions from amorphous calcium phosphate and poly (L-lactic acid)-based Electrospinning nanofibrous scaffold. Sci. Rep. 2017, 7, 45655. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of Electrospinning and Electro-spun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Alborzi, S.; Lim, L.-T.; Kakuda, Y. Encapsulation of folic acid and its stability in sodium alginate-pectin-poly (ethylene oxide) Electro-spun fibres. J. Microencapsul. 2013, 30, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and Electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpoor, E.; Mohammadi, A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng. 2015, 165, 149–155. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghorbani, M.; Jafari, S.M.; Sadeghi Mahoonak, A.; Rajabzadeh, G. Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocoll. 2015, 51, 327–337. [Google Scholar] [CrossRef]
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food chem. 2018, 268, 86–93. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Lagaron, J.M.; López-Rubio, A. Development and characterization of food-grade Electro-spun fibers from amaranth protein and pullulan blends. Food Res. Int. 2013, 54, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Moomand, K.; Lim, L.-T. Oxidative stability of encapsulated fish oil in Electro-spun zein fibres. Food Res. Int. 2014, 62, 523–532. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Lagaron, J.M.; López-Rubio, A. Photoprotection of folic acid upon encapsulation in food-grade amaranth (Amaranthus hypochondriacus L.) protein isolate–Pullulan Electro-spun fibers. LWT-Food Sci. Technol. 2015, 62, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.A.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial Electro-spun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C 2019, 94, 393–402. [Google Scholar] [CrossRef]
- Selim, K.A.; Khalil, K.E.; Abdel-Bary, M.S.; Abdel-Azeim, N.A. Extraction, encapsulation and utilization of red pigments from Roselle (Hibiscus sabdariffa L.) as natural food colourants. In Proceedings of the 5th Alexandria Conference of Food and Dairy Science and Technology, Alexandria, Egypt, 4–6 March 2008; pp. 7–20. [Google Scholar]
- Hollingworth, T.; Wekell, M.M. Paralytic shellfish poison biological method, final action. In Official Methods of Analysis of AOAC; Hellrich, K., Ed.; AOAC: Arlington, VA, USA, 1990; pp. 881–882. [Google Scholar]
- Wang, W.-D.; Xu, S.-Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Kaushik, V.; Roos, Y.H. Limonene encapsulation in freeze drying of gum Arabic–sucrose–gelatin systems. LWT-Food Sci. Technol. 2007, 40, 1381–1391. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC Electro-spun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electro-spun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.-T. Electro-spun soy protein isolate-based fiber fortified with anthocyanin-rich red raspberry (Rubus strigosus) extracts. Food Res. Int. 2013, 52, 467–472. [Google Scholar] [CrossRef]
- Khazaei, K.M.; Jafari, S.; Ghorbani, M.; Kakhki, A.H. Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr. Polym. 2014, 105, 57–62. [Google Scholar] [CrossRef]
- Hogan, S.A.; McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Emulsification and microencapsulation properties of sodium caseinate/carbohydrate blends. Int. Dairy J. 2001, 11, 137–144. [Google Scholar] [CrossRef]
- Chranioti, C.; Nikoloudaki, A.; Tzia, C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: Incorporation in a chewing gum system. Carbohydr. Polym. 2015, 127, 252–263. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using Electrospinning process—A review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Meng, G.T.; Ma, C.Y. Thermal properties of Phaseolus angularis (red bean) globulin. Food Chem. 2001, 73, 453–460. [Google Scholar] [CrossRef]
- Ma, C.Y.; Harwalkar, V.R. Studies of thermal denaturation of oat globulin by differential scanning calorimetry. J. Food Sci. 1988, 53, 531–534. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Pardo, J.E.; López, E.; Alvarruiz, A.; Alonso, G.L. Influence of different drying and aging conditions on saffron constituents. J. Agric. Food Chem. 2005, 53, 3974–3979. [Google Scholar] [CrossRef] [PubMed]
- Tsimidou, M.; Biliaderis, C.G. Kinetic studies of saffron (Crocus sativus L.) quality deterioration. J. Agric. Food Chem. 1997, 45, 2890–2898. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Fabrication and characterization of Electro-spun gelatin nanofibers crosslinked with oxidized phenolic compounds. Int. J. Biol. Macromol. 2017, 103, 1062–1068. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the Electro-spun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Jalaja, K.; James, N.R. Electro-spun gelatin nanofibers: A facile cross-linking approach using oxidized sucrose. Int. J. Biol. Macromol. 2015, 73, 270–278. [Google Scholar] [CrossRef]
- Haroun, A.; El Toumy, S. Effect of natural polyphenols on physicochemical properties of crosslinked gelatin-based polymeric biocomposite. J. Appl. Polym. Sci. 2010, 116, 2825–2832. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef]
- Andersen, A.B.; Risbo, J.; Andersen, M.L.; Skibsted, L.H. Oxygen permeation through an oil-encapsulating glassy food matrix studied by ESR line broadening using a nitroxyl spin probe. Food Chem. 2000, 70, 499–508. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D. Effects of processing and storage on food carotenoids. Sight Life Newsl. 2002, 3, 25–35. [Google Scholar]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on microencapsulation of lycopene by spray-drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Silva, D.F.; Favaro-Trindade, C.S.; Rocha, G.A.; Thomazini, M. Microencapsulation of lycopene by gelatin–pectin complex coacervation. J. Food Process. Preserv. 2012, 36, 185–190. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Encapsulation of nanoparticles of d-limonene by spray drying: Role of emulsifiers and emulsifying techniques. Dry. Technol. 2007, 25, 1069–1079. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]

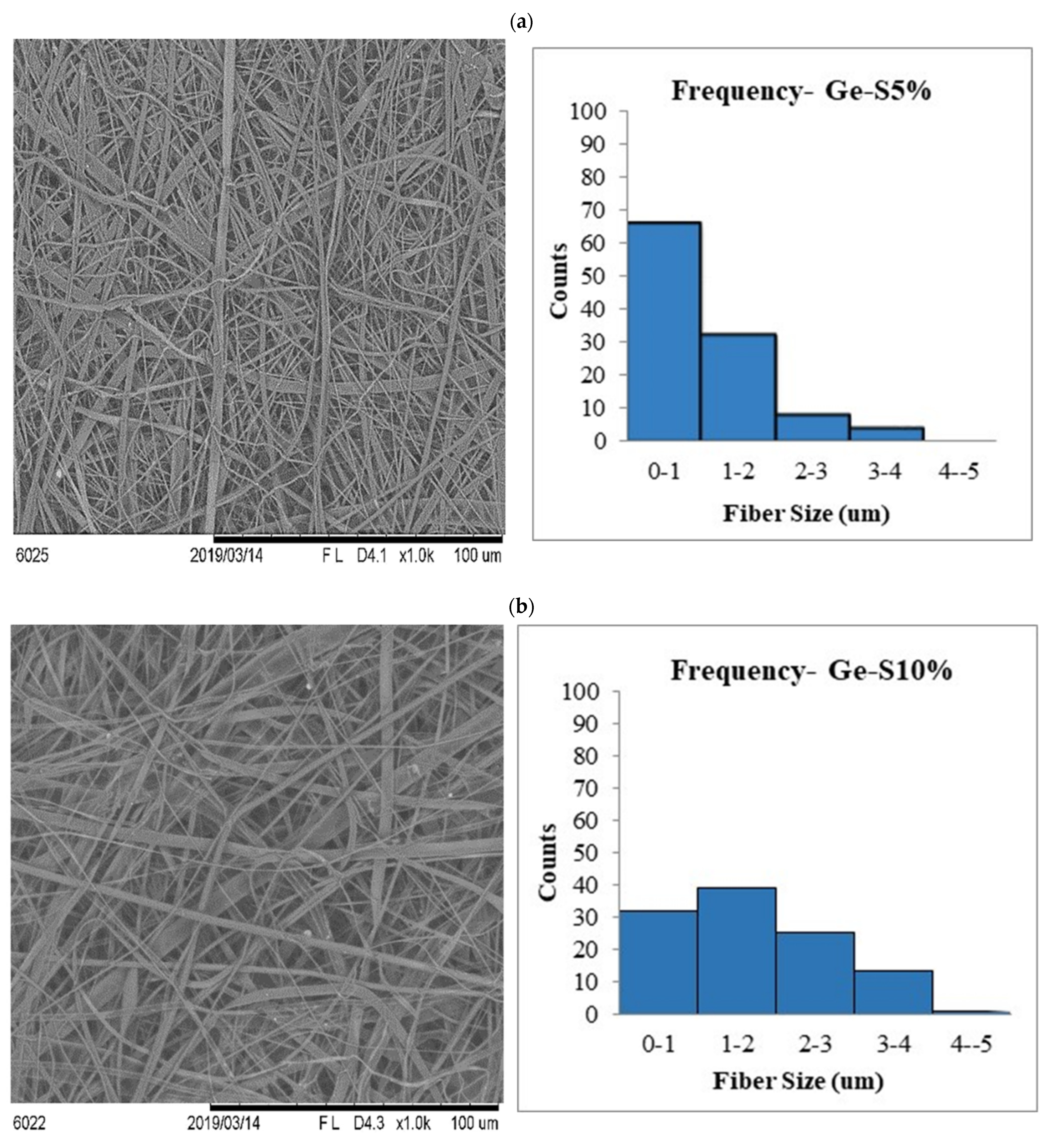

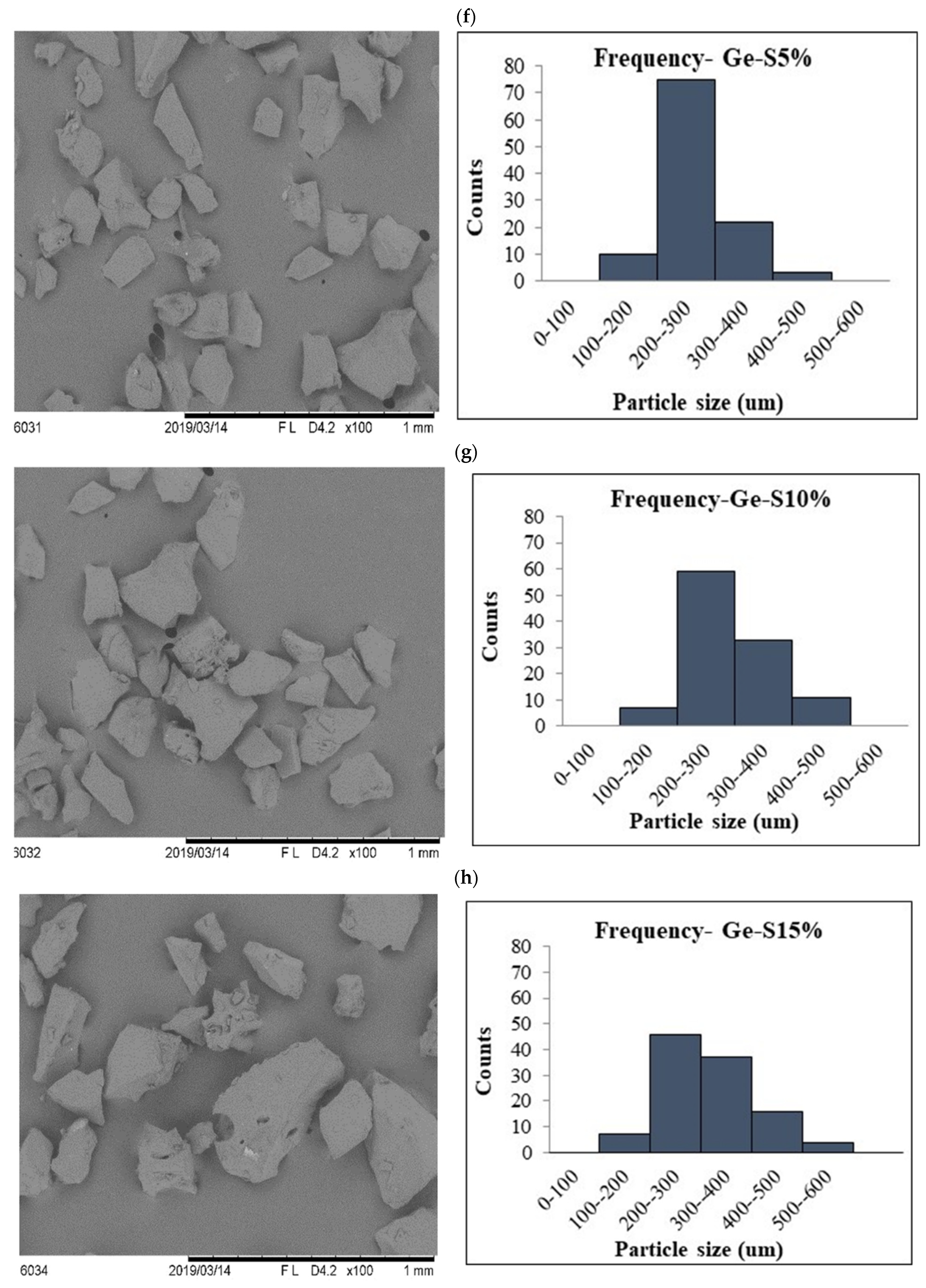

| Encapsulation Technique | Sample | Diameter (um) | Viscosity (Pa.s) |
|---|---|---|---|
| Electrospinning | Ge fiber | 0.75 ± 0.34 d | 1.87 ± 0.02 a |
| Ge-S5% | 1.05 ± 0.69 c | 1.90 ± 0.03 a | |
| Ge-S10% | 1.70 ± 0.95 b | 1.98 ± 0.02 a | |
| Ge-S15% | 2.04 ± 1.07 a | 1.98 ± 0.05 a | |
| Freeze drying | Ge particle | 152 ± 40.6 b | 1.87 ± 0.02 a |
| Ge-S5% | 163 ± 57.2 b | 1.90 ± 0.03 a | |
| Ge-S10% | 195 ± 68.7 a | 1.98 ± 0.02 a | |
| Ge-S15% | 217 ± 86.1 a | 1.98 ± 0.05 a |
| Encapsulation Technique | Sample | EE% of Picrocrocin | EE% of Safranal | EE% of Crocin |
|---|---|---|---|---|
| Electrospinning | Ge-S5% | 71.2 ± 1.36 aA | 63.5 ± 0.55 aB | 68.1 ± 0.78 aC |
| Ge-S10% | 79.0 ± 0.95 bA | 67.8 ± 1.06 bC | 71.5 ± 0.66 bB | |
| Ge-S15% | 86.0 ± 1.00 cA | 74.2 ± 0.68 cB | 76.3 ± 1.52 cB | |
| Freeze drying | Ge-S5% | 62.6 ± 0.50 cA | 51.7 ± 1.15 cC | 59.2 ± 1.00 cB |
| Ge-S10% | 68.7 ± 0.57 bA | 59.7 ± 2.08 bB | 61.6 ± 0.53 bB | |
| Ge-S15% | 74.7 ± 1.15 aA | 65.8 ± 0.76 aC | 69.0 ± 0.95 aB |
| Encapsulation Technique | Sample | Tm (°C) | Td (°C) | ΔH (J/g) |
|---|---|---|---|---|
| Electrospinning | Saffron extract | 32.1 ± 2.34 b | 36.1 ± 3.31 c | 27.1 ± 1.66 d |
| Ge fiber | 46.5 ± 4.06 c | 87.7 ± 5.73 b | 415 ± 8.66 a | |
| Ge-S5% | 55.4 ± 2.54 bc | 90.1 ± 6.35 b | 340 ± 19.9 c | |
| Ge-S10% | 65.7 ± 5.27 ab | 99.4 ± 5.72 ab | 355 ± 10.1 bc | |
| Ge-S15% | 78.3 ± 7.63 a | 108 ± 7.31 a | 386 ± 5.22 ab | |
| Freeze drying | Saffron extract | 32.1 ± 2.34 b | 36.1 ± 3.31 c | 27.1 ± 1.66 d |
| Ge particle | 36.7 ± 3.89 b | 73.8 ± 3.77 b | 86.0 ± 4.55 a | |
| Ge-S5% | 42.2 ± 5.78 b | 79.1 ± 5.65 ab | 76.1 ± 4.45 ab | |
| Ge-S10% | 57.9 ± 6.43 a | 89.3 ± 10.1 ab | 66.5 ± 5.22 bc | |
| Ge-S15% | 65.4 ± 4.62 a | 93.2 ± 7.25 a | 61.2 ± 3.47 c |
| Encapsulation Technique | Sample | Temperature (°C) | t½ (day) | (k × 10−2) (min−1) | R2 |
|---|---|---|---|---|---|
| Electrospinning | Control | 4 | 22.4 | 3.1 | 0.98 |
| Ge-S5% | 4 | 53.3 | 1.3 | 0.94 | |
| Ge-S10% | 4 | 77 | 0.9 | 1 | |
| Ge-S15% | 4 | 138 | 0.5 | 0.95 | |
| Control | 24 | 21 | 3.3 | 0.96 | |
| Ge-S5% | 24 | 40 | 1.7 | 1 | |
| Ge-S10% | 24 | 49.5 | 1.4 | 1 | |
| Ge-S15% | 24 | 86.6 | 0.8 | 1 | |
| Control | 35 | 18.7 | 3.7 | 1 | |
| Ge-S5% | 35 | 31.5 | 2.2 | 1 | |
| Ge-S10% | 35 | 38.5 | 1.8 | 1 | |
| Ge-S15% | 35 | 63 | 1.1 | 0.99 | |
| Freeze drying | Control | 4 | 22.4 | 3.1 | 0.98 |
| Ge-S5% | 4 | 43.3 | 1.6 | 1 | |
| Ge-S10% | 4 | 57.8 | 1.2 | 0.98 | |
| Ge-S15% | 4 | 77 | 0.9 | 0.97 | |
| Control | 24 | 21 | 3.3 | 0.96 | |
| Ge-S5% | 24 | 34.7 | 2 | 0.97 | |
| Ge-S10% | 24 | 43.3 | 1.6 | 0.97 | |
| Ge-S15% | 24 | 49.5 | 1.4 | 0.97 | |
| Control | 35 | 18.7 | 3.7 | 0.98 | |
| Ge-S5% | 35 | 27.7 | 2.5 | 0.97 | |
| Ge-S10% | 35 | 33 | 2.1 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golpira, F.; Maftoonazad, N.; Ramaswamy, H.S. Evaluation of Freeze Drying and Electrospinning Techniques for Saffron Encapsulation and Storage Stability of Encapsulated Bioactives. J. Compos. Sci. 2021, 5, 326. https://doi.org/10.3390/jcs5120326
Golpira F, Maftoonazad N, Ramaswamy HS. Evaluation of Freeze Drying and Electrospinning Techniques for Saffron Encapsulation and Storage Stability of Encapsulated Bioactives. Journal of Composites Science. 2021; 5(12):326. https://doi.org/10.3390/jcs5120326
Chicago/Turabian StyleGolpira, Fatemeh, Neda Maftoonazad, and Hosahalli S. Ramaswamy. 2021. "Evaluation of Freeze Drying and Electrospinning Techniques for Saffron Encapsulation and Storage Stability of Encapsulated Bioactives" Journal of Composites Science 5, no. 12: 326. https://doi.org/10.3390/jcs5120326





