Study on Indium (III) Oxide/Aluminum Thermite Energetic Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Al/In2O3 Energetic Composites
2.2. Characterization Techniques
3. Results and Discussion
3.1. Characterization of the Al/In2O3 Energetic Composite Materials
3.2. Sensitivity Properties of the Al/In2O3 Energetic Composite Materials
3.3. Ignition Ability of the Al/In2O3 Energetic Composite Materials
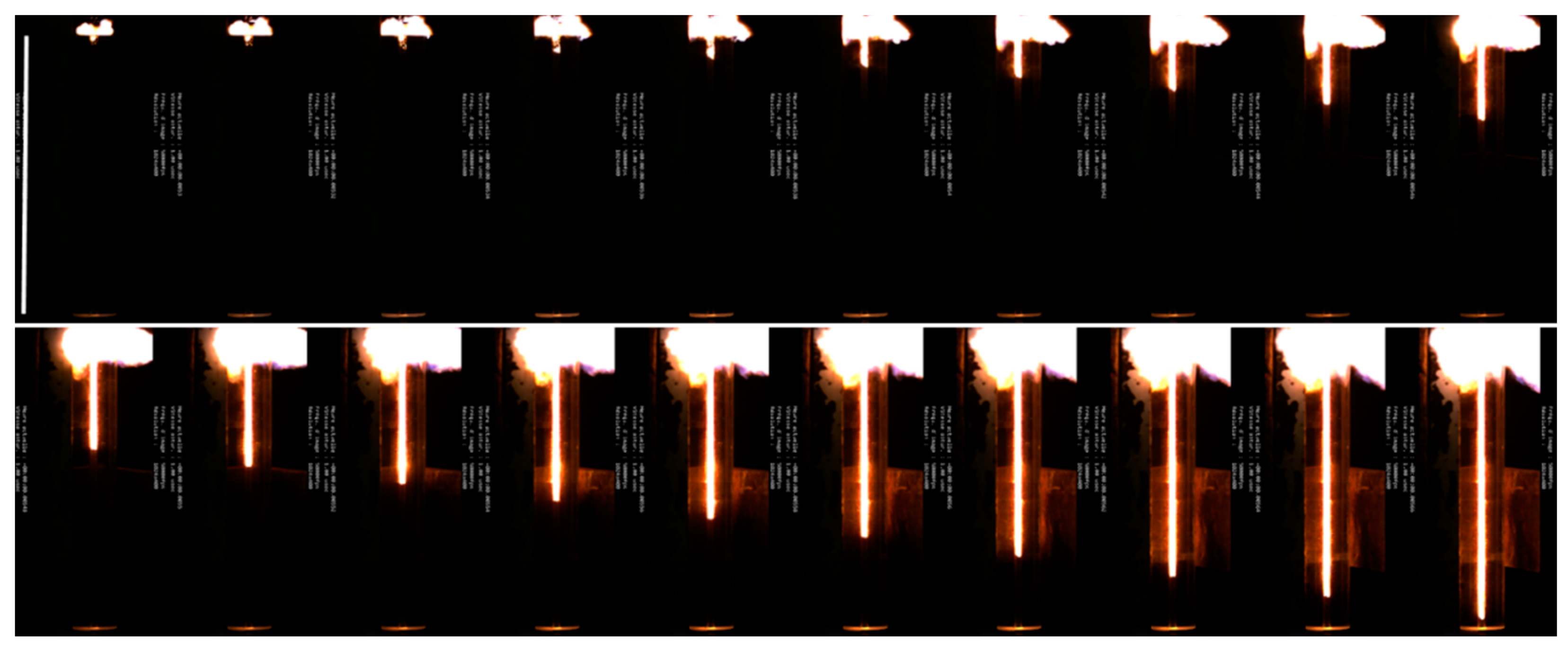
3.4. Combustion Velocity of the Al/In2O3_nm Energetic Composite Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fischer, S.; Grubelich, M. Theoretical Energy Release of Thermites, Intermetallics, and Combustible Metals. In Proceedings of the 24th International Pyrotechnics Seminar, Monterey, CA, USA, 27–31 July 1998; pp. 231–286. [Google Scholar]
- Levitas, V.I.; Pantoya, M.L.; Dikici, B. Melt-dispersion mechanism for fast reaction of aluminium particles: Extension for micron scale particles and fluorination. Appl. Phys. Lett. 2008, 92, 011921. [Google Scholar] [CrossRef] [Green Version]
- Walter, K.C.; Pesiri, D.R.; Wilson, D.E. Manufacturing and Performance of Nanometric Al/MoO3 Energetic Materials. J. Propuls. Power 2007, 23, 645–650. [Google Scholar] [CrossRef]
- Son, S.F.; Asay, B.W.; Foley, T.J.; Yetter, R.A.; Wu, M.H.; Risha, G.A. Combustion of nanoscale Al/MoO3 thermite in microchannels. J. Propuls. Power 2007, 23, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Dikici, B.; Pantoya, M.L.; Levitas, V. The effect of pre-heating on flame propagation in nanocomposite thermites. Combust. Flame 2010, 157, 1581–1585. [Google Scholar] [CrossRef]
- Clark, B.R.; Pantoya, M.L.; Hunt, E.M.; Kelly, T.J.; Allen, B.F.; Heaps, R.J.; Daniels, M.A. Synthesis and characterization of flexible, free-standing, energetic thin films. Surf. Coatings Technol. 2015, 284, 422–426. [Google Scholar] [CrossRef]
- Zakiyyan, N.A.; Wang, R.; Thiruvengadathan, C.; Staley, J.M.; Gangopadhyay, K.; Maschmann, M.R.; Gangopadhyay, S. Combustion of aluminum nanoparticles and exfoliated 2D molybdenum trioxide composites. Combust. Flame 2018, 187, 1–10. [Google Scholar] [CrossRef]
- Sanders, V.E.; Asay, B.W.; Foley, T.J.; Tappan, B.C.; Pacheco, A.N.; Son, S.F. Reaction propagation of four nanoscale energetic composites (Al/MoO3, Al/WO3, Al/CuO and Bi2O3). J. Propuls. Power 2007, 23, 707–714. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Chiou, W.A.; Fiore, R.; Zachariah, M.R. In Situ microscopy of rapidly heated nano-Al and nano-Al/WO3 thermites. Appl. Phys. Lett. 2010, 97, 133104. [Google Scholar] [CrossRef] [Green Version]
- Gibot, P.; Bach, A.; Vidal, L.; Schnell, F.; Gadiou, R.; Spitzer, D. Safer and performing energetic materials based on polyani-line-doped nanocomposites. J. Energetic Mater. 2017, 35, 136–147. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermite foams: From nanopowder to object. Chem. Eng. J. 2017, 316, 807–812. [Google Scholar] [CrossRef]
- Prakash, A.; McCormick, A.V.; Zachariah, M.R. Aero-sol-gel synthesis of nanoporous iron-oxide particles: A potential oxidiz-er for nanoenergetic materials. Chem. Mater. 2004, 16, 1466–1471. [Google Scholar] [CrossRef]
- Plantier, K.B.; Pantoya, M.L.; Gash, A.E. Combustion wave speeds of nanocomposite Al/Fe2O3: The effects of Fe2O3 particle synthesis technique. Combust. Flame 2005, 140, 299–309. [Google Scholar] [CrossRef]
- Bezmelnitsyn, A.R.; Thiruvengadathan, S.; Barizuddin, D.; Tappmeyer, S.; Apperson, K.; Gangopadhyay, S. Modified nanoenergetic composites with tunable combustion characteristics for propellant applications. Propellants Explos. Pyro-Tech. 2010, 35, 384–394. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, B.; Shen, R.; Ye, J.; Thomas, J.A.; Chao, Y. Significantly Enhanced Energy Output from 3D Ordered Macroporous Structured Fe2O3/Al Nanothermite Film. ACS Appl. Mater. Interfaces 2012, 5, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liao, X.; Xiao, L.; Jian, X.; Zhou, W. High-Energy Pollen-Like Porous Fe2O3 /Al Thermite: Synthesis and Properties. Propellants Explos. Pyrotech. 2015, 40, 867–872. [Google Scholar] [CrossRef]
- Petrantoni, M.; Rossi, C.; Conédéra, V.; Bourrier, D.; Pierre, A.; Tenailleau, C. Synthesis process of nanowired Al/CuO thermite. J. Phys. Chem. Solids. 2010, 71, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Shen, R.; Ye, Y.; Zhu, P.; Hu, Y.; Wu, L. Influence of Al/CuO reactive multilayer films additives on exploding foil initiator. J. Appl. Phys. 2011, 110, 094505. [Google Scholar] [CrossRef]
- Yan, S.; Jian, G.; Zachariah, M.R. Electrospun Nanofiber-Based Thermite Textiles and their Reactive Properties. ACS Appl. Mater. Interfaces 2012, 4, 6432–6435. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Kuntz, J.D.; Gash, A.E. Electrophoretic deposition and mechanistic studies of nano-Al/CuO thermites. J. Appl. Phys. 2012, 112, 024316. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Kuntz, J.D.; Gash, A.E. The role of fuel particle size on flame propagation velocity in thermites with a nanoscale oxidizer. Propellants Explos. Pyrotech. 2014, 39, 407–415. [Google Scholar] [CrossRef]
- McCollum, J.; Pantoya, M.L.; Iacono, S.T. Activating aluminum reactivity with fluoropolymer coatings for improved energetic composite combustion. ACS Appl. Mater. Interfaces 2015, 7, 18742–18749. [Google Scholar] [CrossRef] [PubMed]
- Piekiel, N.W.; Zhou, L.; Sullivan, K.T.; Chowdhury, S.; Egan, G.C.; Zachariah, M.R. Initiation and Reaction in Al/Bi2O3 nanothermites: Evidence for the predominance of condensed phase chemistry. Combust. Sci. Technol. 2014, 186, 1209–1224. [Google Scholar] [CrossRef]
- Nellums, R.R.; Terry, C.B.; Tappan, C.B.; Son, S.F.; Groven, L.J. Effect of solids loading on resonant mixed Al-Bi2O3 nanothermite powders. Propellants Explos. Pyrotech. 2013, 38, 605–610. [Google Scholar] [CrossRef]
- Wang, L.; Luss, D.; Martirosyan, K.S. The behavior of nanothermite reaction based on Bi2O3/Al. J. Appl. Phys. 2011, 110, 74311. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.; Zachariah, M.R. Simultaneous pressure and optical measurements of nano aluminium thermites: Investigating the reaction mechanism. J. Propuls. Power 2010, 26, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Gibot, P.; Goetz, V. Aluminium/tin (IV) oxide thermite composite: Sensitivities and reaction propagation. J. Energetic Mater. 2020, 38, 295–308. [Google Scholar] [CrossRef]
- Downs, A.J. Chemistry of Aluminium, Gallium, Indium, and Thallium, 1st ed.; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Shimada, S.; Sato, O.; Tsunashima, A.; Kodaira, K. Crystallization of In2O3 by vapour reaction. J. Cryst. Growth 1987, 80, 366–370. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Micali, G.; Rizzo, G.; Callone, E.; Carturan, G. Resistive CO gas sensors based on In2O3 and InSnOx nanopowders synthesized via starch-aided sol–gel process for automotive applications. Sens. Actuators B Chem. 2008, 132, 224–233. [Google Scholar] [CrossRef]
- Zhan, Z.; Jiang, D.; Xu, J. Investigation of a new In2O3-based selective H2 gas sensor with low power consumption. Mater. Chem. Phys. 2005, 90, 250–254. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Shen, J. Hydrothermal synthesis of In2O3 for detecting H2S in air. Sens. Actuators B Chem. 2006, 115, 642–646. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, Z.; Pan, Q.; Xu, J.; Xiang, Q.; Yu, W.; Chu, Y. High aspect ratio In2O3 nanowires: Synthesis, mechanism and NO2 gas-sensing properties. Sens. Actuators B Chem. 2008, 130, 802–808. [Google Scholar] [CrossRef]
- Song, P.; Wang, Q.; Yang, Z. Biomorphic synthesis and gas response of In2O3 microtubules using cotton fibres as templates. Sens. Actuators B 2012, 168, 421–428. [Google Scholar] [CrossRef]
- Wagner, T.; Sauerwald, T.; Kohl, C.-D.; Waitz, T.; Weidmann, C.; Tiemann, M. Gas sensor based on ordered mesoporous In2O3. Thin Solid Films 2009, 517, 6170–6175. [Google Scholar] [CrossRef]
- Talawar, M.; Agrawal, A.; Anniyappan, M.; Wani, D.; Bansode, M.; Gore, G. Primary explosives: Electrostatic discharge initiation, additive effect and its relation to thermal and explosive characteristics. J. Hazard. Mater. 2006, 137, 1074–1078. [Google Scholar] [CrossRef]
- Greason, W.D. Electrostatic discharge characteristics for the human body and circuit packs. J. Electrost. 2003, 59, 285–300. [Google Scholar] [CrossRef]
- Puszynski, J.A.; Bulian, C.J.; Swiatkiewicz, J.J. Processing and ignition characteristics of aluminum-bismuth trioxide nano-thermite system. J. Propuls. Power 2007, 23, 698–706. [Google Scholar] [CrossRef]
- Steelman, R.; Clark, B.; Pantoya, M.L.; Heaps, R.J.; Daniels, M.A. Desensitizing nano powders to electrostatic discharge ignition. J. Electrost. 2015, 76, 102–107. [Google Scholar] [CrossRef]
- Poper, K.H.; Collins, E.S.; Pantoya, M.L.; Daniels, M.A. Controlling the electrostatic discharge ignition sensitivity of composite energetic materials using carbon nanotube additives. J. Electrost. 2014, 72, 428–432. [Google Scholar] [CrossRef]
- Foley, T.; Pacheco, A.; Malchi, J.; Yetter, R.; Higa, K. Development of Nanothermite Composites with Variable Electrostatic Discharge Ignition Thresholds. Propellants Explos. Pyrotech. 2007, 32, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Bach, A.; Gibot, P.; Vidal, L.; Gadiou, R.; Spitzer, D. Modulation of the Reactivity of a WO3/Al Energetic Material with Graphitized Carbon Black as Additive. J. Energetic Mater. 2015, 33, 260–276. [Google Scholar] [CrossRef]
- Kelly, D.G.; Beland, P.; Brousseau, P.; Petre, C.F. Electrostatic discharge sensitivity and resistivity measurements of Al nanothermites and their fuel and oxidant precursors. Cent. Eur. J. Energetic Mater. 2017, 14, 105–119. [Google Scholar] [CrossRef]
- Siegert, B.; Comet, M.; Muller, O.; Pourroy, G.; Spitzer, D. Reduced-Sensitivity Nanothermites Containing Manganese Oxide Filled Carbon Nanofibers. J. Phys. Chem. C 2010, 114, 19562–19568. [Google Scholar] [CrossRef]
- Collins, E.S.; Skelton, B.R.; Pantoya, M.L.; Irin, F.; Green, M.J. Ignition sensitivity and electrical conductivity of an aluminum fluoropolymer reactive material with carbon nanofillers. Combust. Flame 2015, 162, 1417–1421. [Google Scholar] [CrossRef]
- Kappagantula, K.; Pantoya, M.L.; Hunt, E.M. Impact ignition of aluminum-teflon based energetic materials impregnated with nano-structured carbon additives. J. Appl. Phys. 2012, 112, 024902. [Google Scholar] [CrossRef]
- Kappagantula, K.; Pantoya, M.L. Experimentally measured thermal transport properties of aluminum? Polytetrafluoroethylene nanocomposites with graphene and carbon nanotube additives. Int. J. Heat Mass Transf. 2012, 55, 817–824. [Google Scholar] [CrossRef]
- Pichot, V.; Comet, M.; Miesch, J.; Spitzer, D. Nanodiamond for tuning the properties of energetic composites. J. Hazard. Mater. 2015, 300, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Gibot, P.; Goetz, V. SnO2—Polyaniline composites for the desensitization of Al/SnO2 thermite composites. J. Appl. Polym. Sci. 2020, 137, 48947. [Google Scholar] [CrossRef]
- Gibot, P.; Miesch, Q.; Bach, A.; Schnell, F.; Gadiou, R.; Spitzer, D. Mechanical Desensitization of an Al/WO3 Nanothermite by Means of Carbonaceous Coatings Derived from Carbohydrates. J. Carbon Res. 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Ohkura, Y.; Rao, P.M.; Zheng, X. Flash ignition of Al nanoparticles: Mechanism and applications. Combust. Flame 2011, 158, 2544–2548. [Google Scholar] [CrossRef]
- Haussonne, J.M. Céramiques Pour L’électronique et L’électrotechnique; EPFL Press: Lauzane, Switzerland, 2002. (In French) [Google Scholar]
- The North Atlantic Treaty Organization. NATO Standardization Agreement (STANAG) on Explosives, Impact Sensitivity Tests, No. 4489, 1st ed.; NATO: Brussels, Belgium, 17 September 1999. [Google Scholar]
- The North Atlantic Treaty Organization. NATO Standardization Agreement (STANAG) on Explosive, Friction Sensitivity Tests, No. 4487, 1st ed.; NATO: Brussels, Belgium, 22 August 2002. [Google Scholar]
- Gibot, P. Templated synthesis of Cr2O3 material for energetic composites with high performance. Solid State Sci. 2019, 94, 162–167. [Google Scholar] [CrossRef]
- Weast, R.C.; Lide, D.R. Handbook of Chemistry and Physics, 68th ed.; Edition CRC Press: Boca Raton, FL, USA, 1986; pp. 1987–1988. [Google Scholar]



| Material | Molar Mass (g/mol) | Density (g/cm3) | ΔH°f (298 K, kJ/mol) |
|---|---|---|---|
| Al | 26.98 | 2.70 | 0 |
| In2O3 | 7.18 | 7.18 | −925.8 |
| Al2O3 | 3.05 | 3.05 | 1675.7 |
| In | 7.31 | 7.31 | 0 |
| Material | σ (S/cm) | Density (TMD %) |
|---|---|---|
| Al | 4.44 × 10−8 | 43.5 |
| In2O3_µm | 2.55 × 10−2 | 41.5 |
| In2O3_nm | 7.15 × 10−3 | 35.4 |
| Al/In2O3_µm | 1.19 × 10−1 | 46.1 |
| Al/In2O3_nm | 7.11 × 10−3 | 41.8 |
| Sensitivity Test | Impact (J) | Friction (N) | ESD (mJ) |
|---|---|---|---|
| Al/In2O3_µm | >100 | >360 | 27.71 |
| Al/In2O3_nm | >100 | 324 | 0.31 |
| Metals | Density (g/cm3) | Melting Point (Tm, °C) | Boiling Point (Tb, °C) | Electrical Conductivity (S/m) | Thermal Conductivity (W/m/K) |
|---|---|---|---|---|---|
| Indium (In) | 7.31 | 156.6 | 2072 | 11.6 | 81.6 |
| Tin (Sn) | 7.29 | 231.9 | 2602 | 9.17 | 66.6 |
| Iron (Fe) | 7.84 | 1538 | 2861 | 9.93 | 80.2 |
| Molybdenum (Mo) | 10.22 | 2623 | 4639 | 18.7 | 138 |
| Tungsten (W) | 19.3 | 3422 | 5555 | 8.9 | 174 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibot, P.; Puel, E. Study on Indium (III) Oxide/Aluminum Thermite Energetic Composites. J. Compos. Sci. 2021, 5, 166. https://doi.org/10.3390/jcs5070166
Gibot P, Puel E. Study on Indium (III) Oxide/Aluminum Thermite Energetic Composites. Journal of Composites Science. 2021; 5(7):166. https://doi.org/10.3390/jcs5070166
Chicago/Turabian StyleGibot, Pierre, and Estelle Puel. 2021. "Study on Indium (III) Oxide/Aluminum Thermite Energetic Composites" Journal of Composites Science 5, no. 7: 166. https://doi.org/10.3390/jcs5070166
APA StyleGibot, P., & Puel, E. (2021). Study on Indium (III) Oxide/Aluminum Thermite Energetic Composites. Journal of Composites Science, 5(7), 166. https://doi.org/10.3390/jcs5070166






