Platinum-Decorated TiO2: One Step Fast Monometallic Impregnation and Plasma Effect on Nanoparticles
Abstract
:1. Introduction
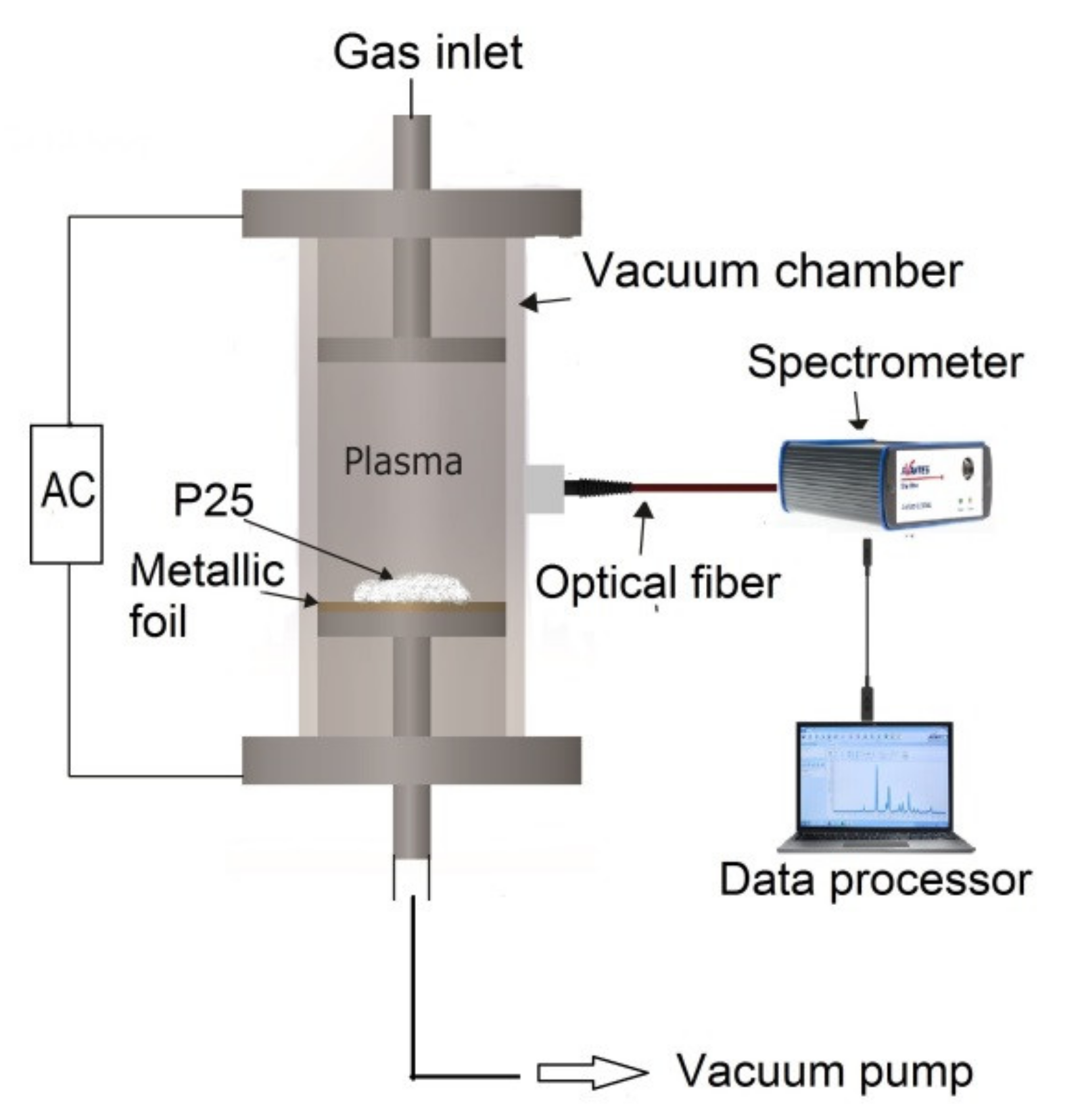
2. Materials and Methods
2.1. Materials
2.2. Characterization
3. Results and Discussion
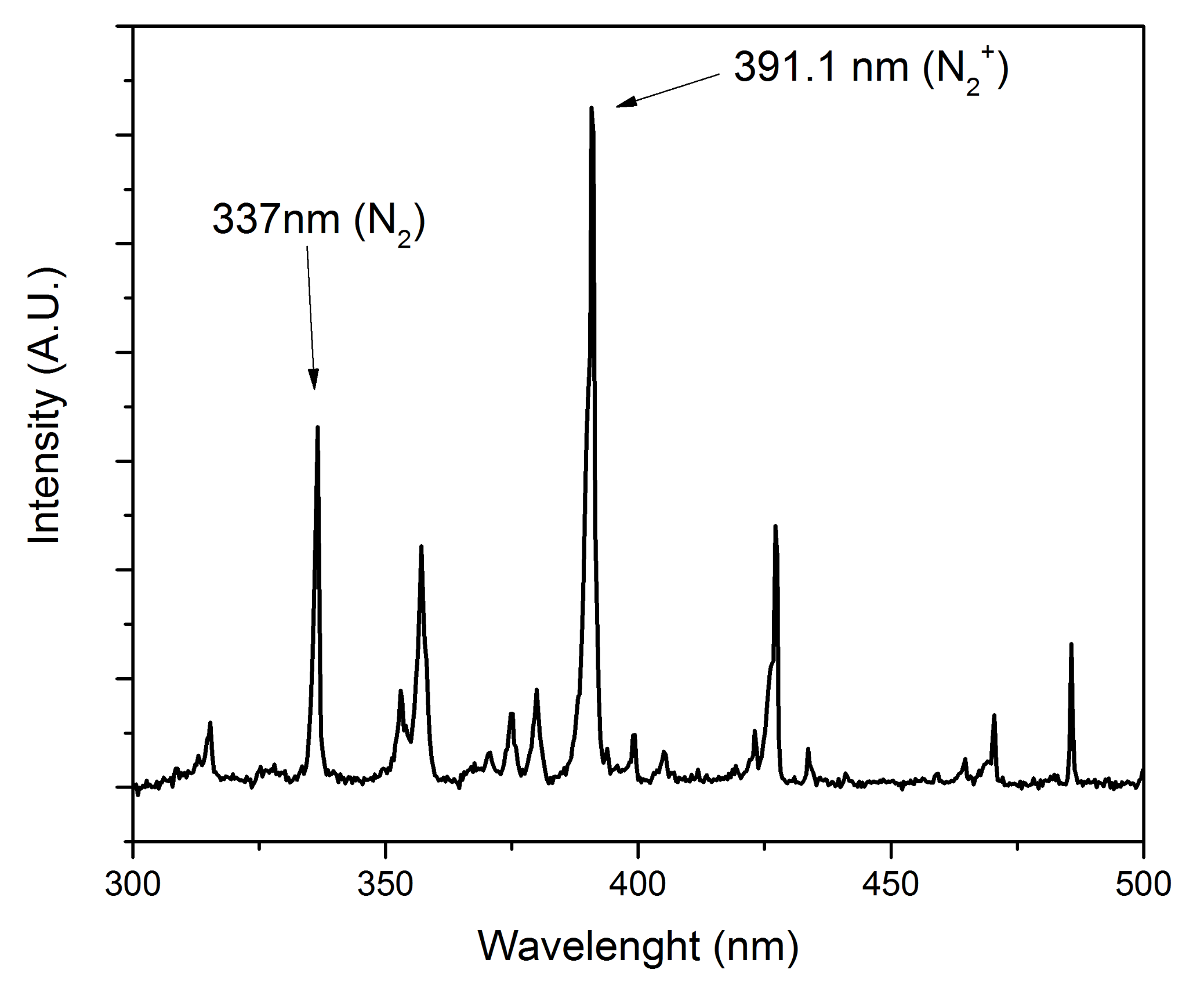
3.1. Optical Emission Spectroscopy (OES)
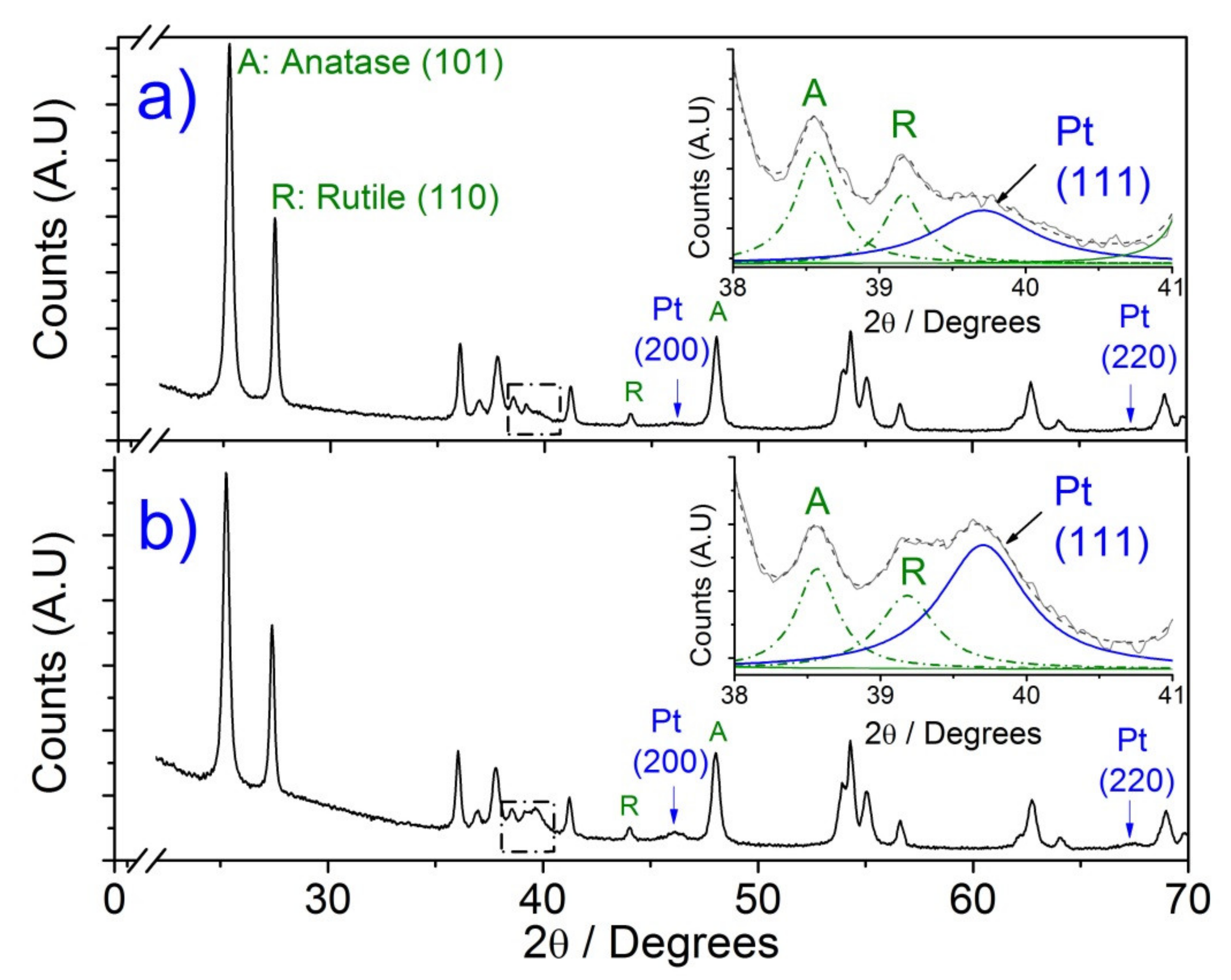
3.2. X-ray Diffraction (XRD)
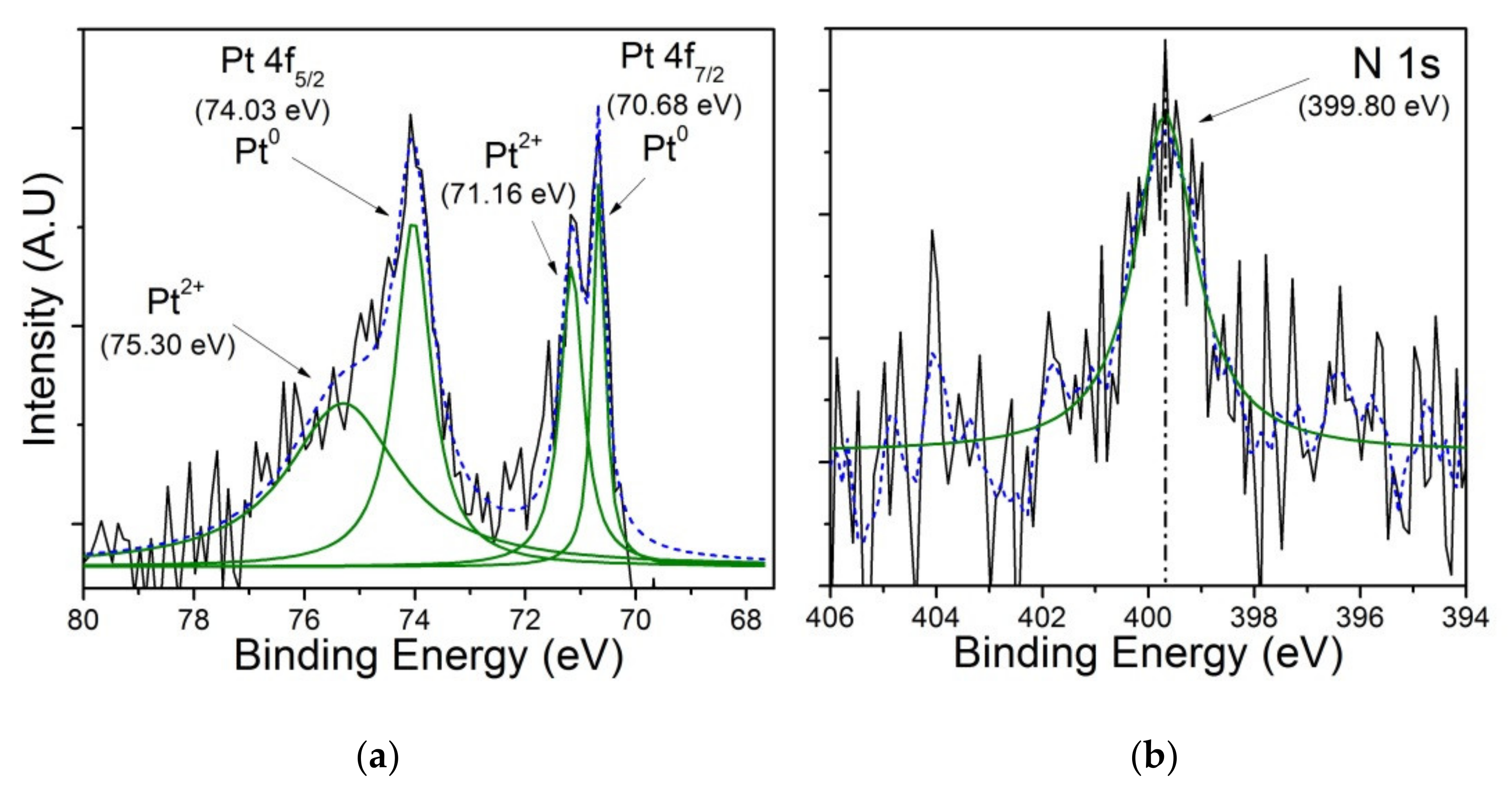
3.3. X-ray Photoelectron Spectroscopy (XPS)
3.4. Diffuse Reflectance Spectroscopy (DRS)
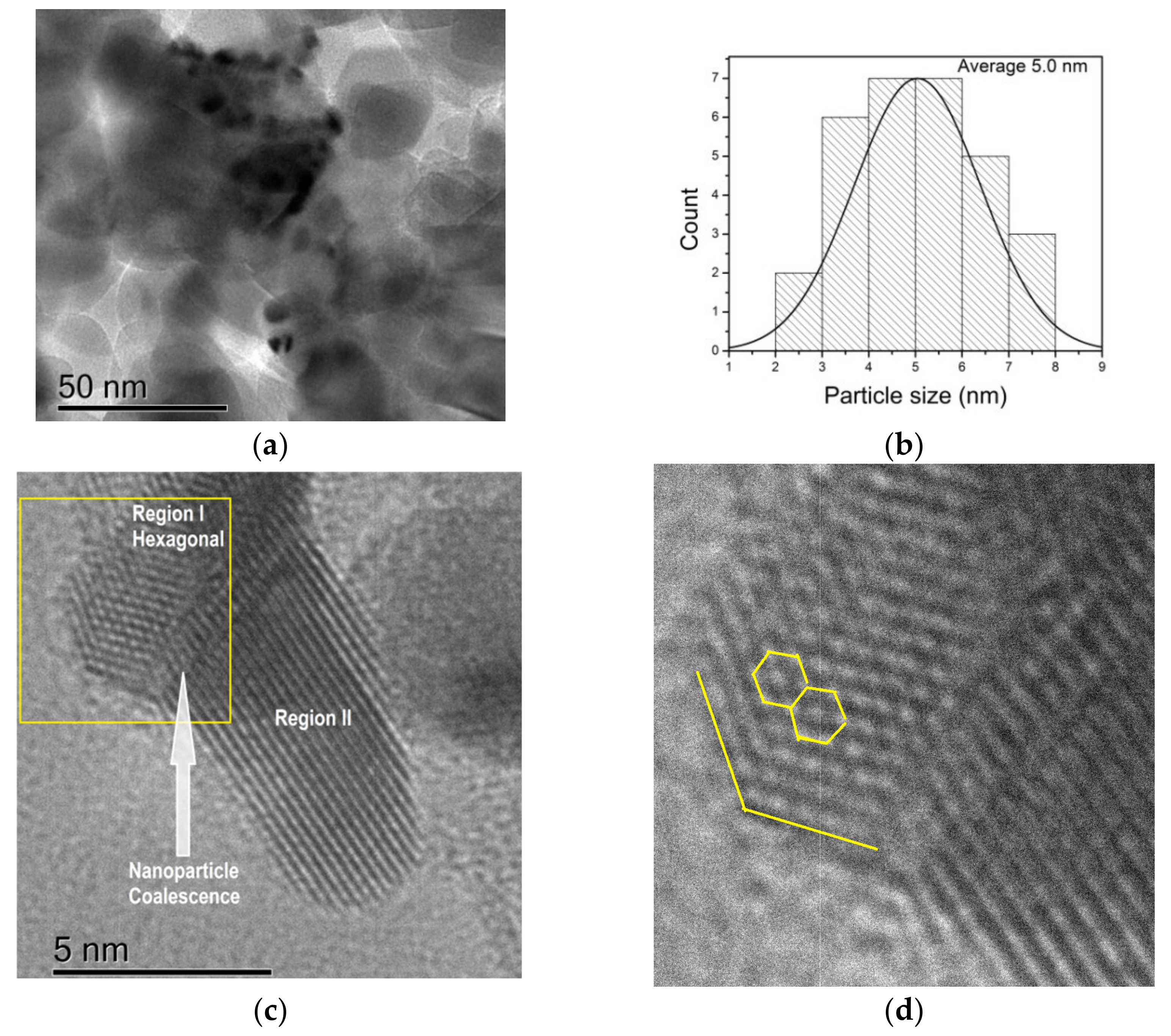
3.5. High-Resolution Transmission Electron Microscopy (HRTEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Liu, W.; Fang, Y.; Xie, J.; Xu, Y. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chin. J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Shen, R.; Xie, J.; Xiang, Q.; Chen, X.; Jiang, J.; Li, X. Ni-based photocatalytic H2-production cocatalysts2. Chin. J. Catal. 2019, 40, 240–288. [Google Scholar] [CrossRef]
- Shen, J.; Wang, R.; Liu, Q.; Yang, X.; Tang, H.; Yang, J. Accelerating photocatalytic hydrogen evolution and pollutant degradation by coupling organic co-catalysts with TiO2. Chin. J. Catal. 2019, 40, 380–389. [Google Scholar] [CrossRef]
- Ikezawa, S.; Homyara, H.; Kubota, T.; Suzuki, R.; Koh, S.; Mutuga, F.; Yoshioka, T.; Nishiwaki, A.; Ninomiya, Y.; Takahashi, M.; et al. Applications of TiO2 film for environmental purification deposited by controlled electron beam-excited plasma. Thin Solid Films 2001, 386, 173–176. [Google Scholar] [CrossRef]
- Calza, P.; Minero, C.; Pelizzetti, A.E. Photocatalytic transformations of chlorinated methanes in the presence of electron and hole scavengers. J. Chem. Soc. Faraday Trans. 1997, 93, 3765–3771. [Google Scholar] [CrossRef]
- Schmelling, D.C.; Gray, K.A.; Kamat, P. V The influence of solution matrix on the photocatalytic degradation of TNT in TiO2 slurries. Water Res. 1997, 31, 1439–1447. [Google Scholar] [CrossRef]
- Zallen, R.; Moret, M.P. The optical absorption edge of brookite TiO2. Solid State Commun. 2006, 137, 154–157. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Lee, D.-S.; Chen, Y.-W. Nano Ag/TiO2 catalyst prepared by chemical deposition and its photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2014, 45, 705–712. [Google Scholar] [CrossRef]
- Rosu, M.; Socaci, C.; Floare-Avram, V.; Borodi, G.; Pogacean, F.; Coros, M.; Măgeruşan, L.; Pruneanu, S. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Rosu, M.-C.; Coros, M.; Pogacean, F.; Magerusan, L.; Socaci, C.; Turza, A.; Pruneanu, S. Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 2017, 70, 13–20. [Google Scholar] [CrossRef]
- Zayadi, R.; Abu Bakar, F. Comparative study on the performance of Au/F-TiO2 photocatalyst synthesized from Zamzam water and distilled water under blue light irradiation. J. Photochem. Photobiol. A Chem. 2017, 346, 338–350. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Scavini, M.; Grunwaldt, J.-D.; Selli, E. One step flame-made fluorinated Pt/TiO2 photocatalysts for hydrogen production. Appl. Catal. B Environ. 2014, 160–161, 144–151. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Hupka, J. Preparation and characterization of Pt/Pd-modified titanium dioxide nanoparticles for visible light irradiation. Catal. Today 2014, 230, 181–187. [Google Scholar] [CrossRef]
- Filippo, E.; Carlucci, C.; Capodilupo, A.L.; Perulli, P.; Conciauro, F.; Corrente, G.A.; Gigli, G.; Ciccarella, G. Enhanced photocatalytic activity of pure anatase TiO2 and Pt-TiO2 nanoparticles synthesized by green microwave assisted route. Mater. Res. 2015, 18, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Cheng, B.; Yu, J.; Ho, W. Halogen poisoning effect of Pt-TiO2 for formaldehyde catalytic oxidation performance at room temperature. Appl. Surf. Sci. 2016, 364, 808–814. [Google Scholar] [CrossRef]
- Jedsukontorn, T.; Saito, N.; Hunsom, M. Photocatalytic behavior of metal-decorated TiO2 and their catalytic activity for transformation of glycerol to value added compounds. Mol. Catal. 2017, 432, 160–171. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Papavasiliou, J.; Deligiannakis, Y.; Konstantinou, I. Photocatalytic performance of Pt-TiO2, Pt-N-TiO2 and Pt-N/F-TiO2 towards simultaneous Cr(VI) reduction/benzoic acid oxidation: Insights into photogenerated charge carrier dynamics and catalyst properties. J. Photochem. Photobiol. A Chem. 2017, 349, 25–35. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, S.; Li, T.; Liu, Q.; Jiang, T.; Guo, Y.; Luo, J.-L. Atomically dispersed Pt on specific TiO2 facets for photocatalytic H2 evolution. J. Catal. 2017, 353, 250–255. [Google Scholar] [CrossRef]
- Trejo-Tzab, R.; Alvarado-Gil, J.J.; Quintana, P. Photocatalytic activity of Degussa P25 TiO2/Au obtained using Argon (Ar) and Nitrogen (N2) plasma. Top. Catal. 2011, 54, 250–256. [Google Scholar] [CrossRef]
- Trejo-Tzab, R.; Alvarado-Gil, J.J.; Quintana, P.; Bartolo-Pérez, P. N-doped TiO2 P25/Cu powder obtained using nitrogen (N2) gas plasma. Catal. Today 2012, 193, 179–185. [Google Scholar] [CrossRef]
- Trejo-Tzab, R.; Caballero-Espada, L.; Quintana, P.; Ávila-Ortega, A.; Medina-Esquivel, R.A. Controlled Phase Changes of Titania Using Nitrogen Plasma. Nanoscale Res. Lett. 2017, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, A.; Zeb, S.; Ali, S.; Waheed, A.; Zakaullah, M. Optical emission spectroscopy of abnormal glow region in nitrogen plasma. Plasma Chem. Plasma Process. 2005, 25, 551–564. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Bazavan, M.; Sugai, H. Langmuir probe diagnostics of electron energy distributions with optical emission spectroscopy in capacitively coupled rf discharge in nitrogen. J. Appl. Phys. 2011, 110, 113303. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Ihnatiuk, D.; Tossi, C.; Tittonen, I.; Linnik, O. Effect of Synthesis Conditions of Nitrogen and Platinum Co-Doped Titania Films on the Photocatalytic Performance under Simulated Solar Light. Catalysts 2020, 10, 1074. [Google Scholar] [CrossRef]
- Naitabdi, A.; Boucly, A.; Rochet, F.; Fagiewicz, R.; Olivieri, G.; Bournel, F.; Benbalagh, R.; Sirotti, F.; Gallet, J.-J. CO oxidation activity of Pt, Zn and ZnPt nanocatalysts: A comparative study by in situ near-ambient pressure X-ray photoelectron spectroscopy. Nanoscale 2018, 10, 6566–6580. [Google Scholar] [CrossRef]
- Matin, A.; Lee, E.; Kim, H.; Yoon, W.; Kwon, Y. Rational syntheses of core–shell Fe@(PtRu) nanoparticle electrocatalysts for the methanol oxidation reaction with complete suppression of CO-poisoning and highly enhanced activity. J. Mater. Chem. A 2015, 3, 17154. [Google Scholar] [CrossRef]
- Viswanathan, B.; Krishanmurthy, K.R. Nitrogen Incorporation in TiO2: Does It Make a Visible Light Photo-Active Material? Int. J. Photoenergy 2012, 2012, 269654. [Google Scholar] [CrossRef] [Green Version]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Michel, J.A.; Morris, W.H.; Lukehart, C.M. Synthesis of shaped Pt nanoparticles using common anions or small molecules as shape-directing agents: Observation of a strong halide or pseudo-halide effect. J. Mater. Chem. A 2015, 3, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.-W.; Kim, I.Y.; Hwang, S.-J.; Ruoff, R.S. One-Pot Synthesis of Platinum Nanoparticles Embedded on Reduced Graphene Oxide for Oxygen Reduction in Methanol Fuel Cells. Electrochem. Solid-State Lett. 2011, 14, B70. [Google Scholar] [CrossRef]
- Grammatikopoulos, P.; Cassidy, C.; Singh, V.; Sowwan, M. Coalescence-induced crystallisation wave in Pd nanoparticles. Sci. Rep. 2015, 4, 5779. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wang, Z.; Li, J. Gold Nanoparticles With Special Shapes: Controlled Synthesis, Surface-enhanced Raman Scattering, and The Application in Biodetection. Sensors 2007, 7, 3299–3311. [Google Scholar] [CrossRef] [Green Version]


| Treatment Time (h) | % Weight | |
|---|---|---|
| Pt | Nitrogen | |
| 1 | 1.6 | 0.38 |
| 2 | 2.9 | 0.47 |
| P25 | TiO2/Pt | ||
|---|---|---|---|
| Power | 100 watts | ||
| Time | 1 h | 2 h | |
| Band Gap (eV) | 3.28 | 3.23 | 3.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trejo-Tzab, R.; Avila-Ortega, A.; Quintana-Owen, P.; Rangel, R.; Álvarez-Lemus, M.A. Platinum-Decorated TiO2: One Step Fast Monometallic Impregnation and Plasma Effect on Nanoparticles. J. Compos. Sci. 2022, 6, 4. https://doi.org/10.3390/jcs6010004
Trejo-Tzab R, Avila-Ortega A, Quintana-Owen P, Rangel R, Álvarez-Lemus MA. Platinum-Decorated TiO2: One Step Fast Monometallic Impregnation and Plasma Effect on Nanoparticles. Journal of Composites Science. 2022; 6(1):4. https://doi.org/10.3390/jcs6010004
Chicago/Turabian StyleTrejo-Tzab, Rudy, Alejandro Avila-Ortega, Patricia Quintana-Owen, Ricardo Rangel, and Mayra Angélica Álvarez-Lemus. 2022. "Platinum-Decorated TiO2: One Step Fast Monometallic Impregnation and Plasma Effect on Nanoparticles" Journal of Composites Science 6, no. 1: 4. https://doi.org/10.3390/jcs6010004






