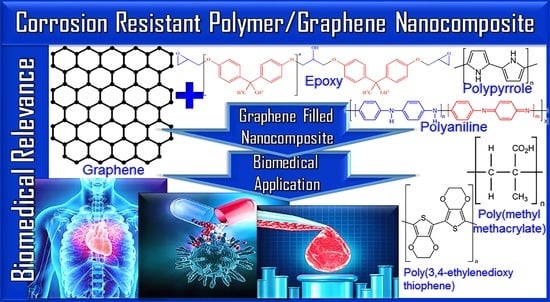
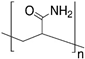
High-Performance Corrosion-Resistant Polymer/Graphene Nanomaterials for Biomedical Relevance
Abstract
:1. Introduction
2. Polymers in Corrosion Resistance
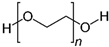
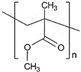
3. Polymeric Nanocomposites for Corrosion Resistance
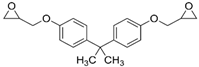
4. Polymer/Graphene Nanocomposite in Corrosion-Resistant Coats
5. Corrosion-Resistant Polymer/Graphene in Biomedical Applications
5.1. In Bioimplants
5.2. Tissue Engineering
5.3. Drug Delivery
6. Encounters and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Omoniyi, P.; Abolusoro, O.; Olorunpomi, O.; Ajiboye, T.; Adewuyi, O.; Aransiola, O.; Akinlabi, E. Corrosion properties of aluminum alloy reinforced with wood particles. J. Compos. Sci. 2022, 6, 189. [Google Scholar] [CrossRef]
- Madan, C.S.; Munuswamy, S.; Joanna, P.S.; Gurupatham, B.G.A.; Roy, K. Comparison of the flexural behavior of high-volume fly ash based concrete slab reinforced with GFRP bars and steel bars. J. Compos. Sci. 2022, 6, 157. [Google Scholar] [CrossRef]
- Boakye, K.; Khorami, M.; Saidani, M.; Ganjian, E.; Dunster, A.; Ehsani, A.; Tyrer, M. Mechanochemical characterisation of calcined impure kaolinitic clay as a composite binder in cementitious mortars. J. Compos. Sci. 2022, 6, 134. [Google Scholar] [CrossRef]
- Malchiodi, B.; Siligardi, C.; Pozzi, P. Unsaturated polyester-based polymer concrete containing recycled cathode ray tube glass aggregate. J. Compos. Sci. 2022, 6, 47. [Google Scholar] [CrossRef]
- Papadatou, M.; Robson, S.; Watts, J.; Dobretsov, S.; Salta, M. Functionality and Composition of Marine Biofilms on Antifouling Coatings. In Proceedings of the Biofilms 9 Conference—Copernicus Meetings, Karlsruhe, Germany, 29 September–1 October 2020. [Google Scholar]
- Yeo, K.; Kim, J.; Kim, J. Development of an anti-corrosion conductive nano carbon coating layer on metal bipolar plates. J. Nanosci. Nanotechnol. 2018, 18, 6278–6282. [Google Scholar] [CrossRef]
- Figueira, R.M.B.B.M.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid sol-gel coatings: Smart and green materials for corrosion mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.-M. Preparation of 2D material dispersions and their applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Joshi, R.; Chughtai, A.A.; Macintyre, C.R. Graphene modified multifunctional personal protective clothing. Adv. Mater. Interfaces 2019, 6, 1900622. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhao, J.; Wang, X.; Xiong, C.; Song, L.; Ding, R.; Han, P.; Li, W. Self-healing performance and corrosion resistance of graphene oxide–mesoporous silicon layer–nanosphere structure coating under marine alternating hydrostatic pressure. Chem. Eng. J. 2019, 361, 792–804. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Aziz, F.; Ismail, A. Spray coating methods for polymer solar cells fabrication: A review. Mater. Sci. Semicond. Process. 2015, 39, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Deng, Y.; Zheng, X.; Bai, Y.; Fang, Y.; Dong, Q.; Wei, H.; Huang, J. Composition engineering in doctor-blading of perovskite solar cells. Adv. Energy Mater. 2017, 7, 1700302. [Google Scholar] [CrossRef]
- Razza, S.; Castro-Hermosa, S.; Di Carlo, A.; Brown, T.M. Research update: Large-area deposition, coating, printing, and processing techniques for the upscaling of perovskite solar cell technology. APL Mater. 2016, 4, 091508. [Google Scholar] [CrossRef] [Green Version]
- Jena, G.; Philip, J. A review on recent advances in graphene oxide-based composite coatings for anticorrosion applications. Prog. Org. Coat. 2022, 173, 107208. [Google Scholar] [CrossRef]
- Upadhyay, D.; Panchal, M.A.; Dubey, R.; Srivastava, V. Corrosion of alloys used in dentistry: A review. Mater. Sci. Eng. A 2006, 432, 1–11. [Google Scholar] [CrossRef]
- Fix, D.; Andreeva, D.V.; Lvov, Y.M.; Shchukin, D.G.; Möhwald, H. Application of inhibitor-loaded halloysite nanotubes in active anti-corrosive coatings. Adv. Funct. Mater. 2009, 19, 1720–1727. [Google Scholar] [CrossRef]
- Koli, D.K.; Agnihotri, G.; Purohit, R. Advanced aluminium matrix composites: The critical need of automotive and aerospace engineering fields. Mater. Today Proc. 2015, 2, 3032–3041. [Google Scholar] [CrossRef]
- Yadav, S.; Gangwar, S.; Singh, S. Micro/nano reinforced filled metal alloy composites: A review over current development in aerospace and automobile applications. Mater. Today Proc. 2017, 4, 5571–5582. [Google Scholar] [CrossRef]
- Ocón, P.; Cristobal, A.; Herrasti, P.; Fatas, E. Corrosion performance of conducting polymer coatings applied on mild steel. Corros. Sci. 2005, 47, 649–662. [Google Scholar] [CrossRef]
- Grujicic, M.; Sellappan, V.; Omar, M.A.; Seyr, N.; Obieglo, A.; Erdmann, M.; Holzleitner, J. An overview of the polymer-to-metal direct-adhesion hybrid technologies for load-bearing automotive components. J. Mater. Process. Technol. 2008, 197, 363–373. [Google Scholar] [CrossRef]
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.; Skirtach, A.G.; Mohan, M. Surface functionalization of chitosan as a coating material for orthopaedic applications: A comprehensive review. Carbohydr. Polym. 2020, 255, 117487. [Google Scholar] [CrossRef] [PubMed]
- Arthur, D.E.; Jonathan, A.; Ameh, P.O.; Anya, C. A review on the assessment of polymeric materials used as corrosion inhibitor of metals and alloys. Int. J. Ind. Chem. 2013, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Talbot, E.D.; Talbot, J.D. Corrosion Science and Technology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Umoren, A.S.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Joulazadeh, M.; Karimi, F. Investigation of corrosion protection performance of epoxy coatings modified by polyaniline/clay nanocomposites on steel surfaces. Prog. Org. Coat. 2014, 77, 347–353. [Google Scholar] [CrossRef]
- Sazou, D.; Deshpande, P.P. Conducting polyaniline nanocomposite-based paints for corrosion protection of steel. Chem. Pap. 2017, 71, 459–487. [Google Scholar] [CrossRef]
- Kausar, A. Conducting Polymer-Based Nanocomposites: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Elkais, A.R.; Gvozdenović, M.M.; Jugović, B.Z.; Grgur, B.N. The influence of thin benzoate-doped polyaniline coatings on corrosion protection of mild steel in different environments. Prog. Org. Coat. 2013, 76, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.K.; Quraishi, M.; Prakash, R. A self-doped conducting polymer “polyanthranilic acid”: An efficient corrosion inhibitor for mild steel in acidic solution. Corros. Sci. 2008, 50, 2867–2872. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Guo, Z.; Guo, N.; Lei, Y.; Chang, X.; Yin, Y. Promoting barrier performance and cathodic protection of zinc-rich epoxy primer via single-layer graphene. Polymers 2018, 10, 591. [Google Scholar] [CrossRef]
- Umoren, S.; Ogbobe, O.; Igwe, I.; Ebenso, E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros. Sci. 2008, 50, 1998–2006. [Google Scholar] [CrossRef]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
- Atta, A.; El-Azabawy, O.; Ismail, H.; Hegazy, M. Novel dispersed magnetite core–shell nanogel polymers as corrosion inhibitors for carbon steel in acidic medium. Corros. Sci. 2011, 53, 1680–1689. [Google Scholar] [CrossRef]
- Tareq, S. Fabrication and Characterisation of Polymeric Nano-Composites; Western Sydney University: Penrith, Australia, 2019. [Google Scholar]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, P.; John, M.J.; Pothen, L.; Sreekala, M.S.; Thomas, S. Natural Fibre and Polymer Matrix Composites and Their Applications in Aerospace Engineering. In Advanced Composite Materials for Aerospace Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–383. [Google Scholar]
- Leygraf, C.; Graedel, T.E.; Tidblad, J.; Wallinder, I.O. Atmospheric Corrosion; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Selvi, S.T.; Raman, V.; Rajendran, N. Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J. Appl. Electrochem. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Zimmerman, A.; Palumbo, G.; Aust, K.; Erb, U. Mechanical properties of nickel silicon carbide nanocomposites. Mater. Sci. Eng. A 2002, 328, 137–146. [Google Scholar] [CrossRef]
- Kumar, S.A.; Meenakshi, K.S.; Sankaranarayanan, T.; Srikanth, S. Corrosion resistant behaviour of PANI–metal bilayer coatings. Prog. Org. Coat. 2008, 62, 285–292. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Recent development in the synthesis of polymer nanocomposites based on nano-alumina. Prog. Polym. Sci. 2015, 51, 74–93. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Siju, C.R.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline–nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Chen, H.; Fan, H.; Su, N.; Hong, R.; Lu, X. Highly hydrophobic polyaniline nanoparticles for anti-corrosion epoxy coatings. Chem. Eng. J. 2021, 420, 130540. [Google Scholar] [CrossRef]
- Fadl, A.; Abdou, M.; Al-Elaa, S.A.; Hamza, M.; Sadeek, S. Evaluation the anti-corrosion behavior, impact resistance, acids and alkali immovability of nonylphenol ethoxylate/TiO2 hybrid epoxy nanocomposite coating applied on the carbon steel surface. Prog. Org. Coat. 2019, 136, 105263. [Google Scholar] [CrossRef]
- Gobara, M.; Baraka, A.; Akid, R.; Zorainy, M. Corrosion protection mechanism of Ce 4+/organic inhibitor for AA2024 in 3.5% NaCl. RSC Adv. 2020, 10, 2227–2240. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2020, 406, 126863. [Google Scholar] [CrossRef]
- Farooq, S.; Razzaq, H.; Razzaque, S.; Khan, B.; Qaisar, S. Structural and physical impacts of nanofillers in ionogels: A comprehensive overview. Polym. Compos. 2019, 40, E11–E23. [Google Scholar] [CrossRef]
- Gu, L.; Ding, J.; Yu, H. Research in graphene-based anticorrosion coatings. Prog. Chem. 2016, 28, 737. [Google Scholar]
- Raman, S.R.; Tiwari, A. Graphene: The thinnest known coating for corrosion protection. JOM 2014, 66, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Kausar, A. Applications of polymer/graphene nanocomposite membranes: A review. Mater. Res. Innov. 2019, 23, 276–287. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, J.; Sun, J.; He, W. Corrosion inhibition of methanol towards stainless steel bipolar plate for direct formic acid fuel cell. Int. J. Hydrog. Energy 2020, 45, 30924–30931. [Google Scholar] [CrossRef]
- Boppana, S.B.; Dayanand, S.; Kumar, A.; Kumar, V.; Aravinda, T. Synthesis and characterization of nano graphene and ZrO2 reinforced Al 6061 metal matrix composites. J. Mater. Res. Technol. 2020, 9, 7354–7362. [Google Scholar] [CrossRef]
- Tatlier, M.; Munz, G.; Fueldner, G.; Henninger, S.K. Effect of zeolite A coating thickness on adsorption kinetics for heat pump applications. Microporous Mesoporous Mater. 2014, 193, 115–121. [Google Scholar] [CrossRef]
- Calovi, M.; Dirè, S.; Ceccato, R.; Deflorian, F.; Rossi, S. Corrosion protection properties of functionalised graphene–acrylate coatings produced via cataphoretic deposition. Prog. Org. Coat. 2019, 136, 105261. [Google Scholar] [CrossRef]
- Mendez, J.A.C.; Escobedo, V.N.M.; Vong, Y.M.; Bueno, J.D.J.P. A review on atmospheric pressure plasma jet and related electrochemical evaluation of corrosion. Green Mater. 2021, 10, 11–22. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Ahmad, S. Recent progress in the synthesis and property enhancement of waterborne polyurethane nanocomposites: Promising and versatile macromolecules for advanced applications. Polym. Rev. 2020, 60, 226–266. [Google Scholar] [CrossRef]
- Zafar, F.; Ghosal, A.; Sharmin, E.; Chaturvedi, R.; Nishat, N. A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog. Org. Coat. 2019, 131, 259–275. [Google Scholar] [CrossRef]
- Bobby, S.; Samad, M.A. Tribological characterization of epoxy hybrid nanocomposite coatings reinforced with graphene oxide and titania. Wear 2020, 466, 203560. [Google Scholar]
- Wahby, M.H.; Atta, A.M.; Moustafa, Y.M.; Ezzat, A.O.; Hashem, A.I. Hydrophobic and superhydrophobic bio-based nano-magnetic epoxy composites as organic coating of steel. Coatings 2020, 10, 1201. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Mahulikar, P.P.; Jadhav, R.S.; Hundiwale, D.G. Performance of polyaniline/TiO2 nanocomposites in epoxy for corrosion resistant coatings. Iran. Polym. J. 2011, 20, 367–376. [Google Scholar]
- Guo, H.; Chao, B.; Zhao, Z.; Nan, D. Preparation of aniline trimer modified graphene oxide new composite coating and study on anticorrosion performance. Mater. Res. Express 2020, 7, 125601. [Google Scholar] [CrossRef]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 15597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabi, M.; Rashed, G.; Zaarei, D. Assessment of graphene oxide/epoxy nanocomposite as corrosion resistance coating on carbon steel. Corros. Eng. Sci. Technol. 2015, 50, 509–516. [Google Scholar] [CrossRef]
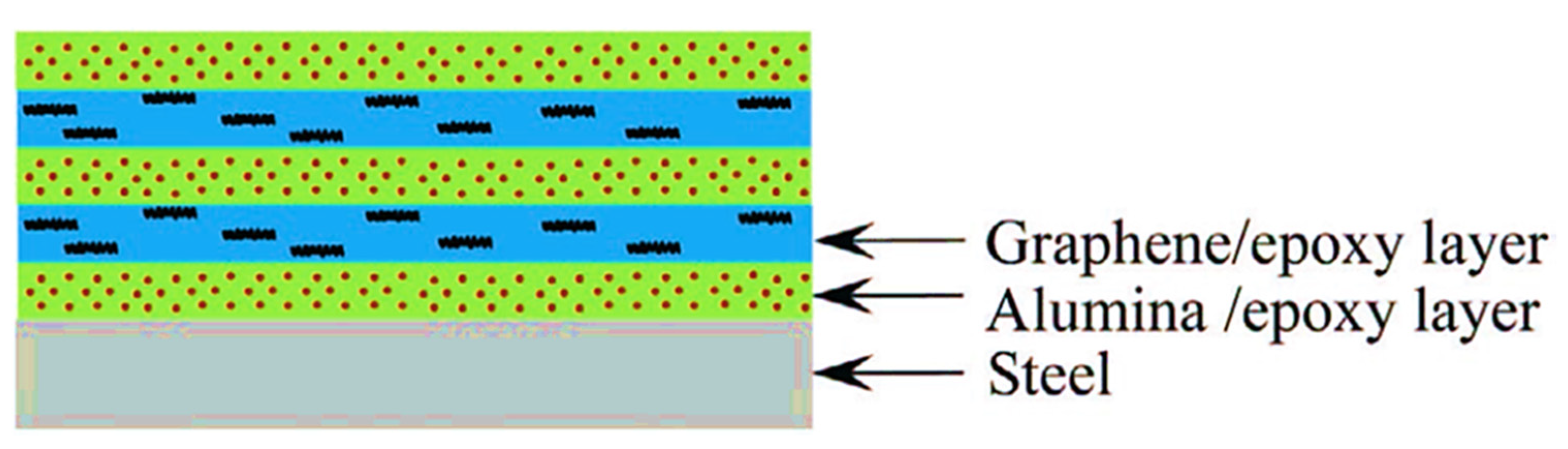
- Zhang, J.; Zhang, W.; Wei, L.; Pu, L.; Liu, J.; Liu, H.; Li, Y.; Fan, J.; Ding, T.; Guo, Z. Alternating multilayer structural epoxy composite coating for corrosion protection of steel. Macromol. Mater. Eng. 2019, 304, 1900374. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Naarmann, H. 1. Introduction. In Science and Applications of Conducting Polymers; Papers from the Sixth European Industrial Workshop; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Bertuoli, P.T.; Baldissera, A.F.; Zattera, A.J.; Ferreira, C.A.; Aleman, C.; Armelin, E. Polyaniline coated core-shell polyacrylates: Control of film formation and coating application for corrosion protection. Prog. Org. Coat. 2019, 128, 40–51. [Google Scholar] [CrossRef]
- Kraljić, M.; Mandić, Z.; Duić, L. Inhibition of steel corrosion by polyaniline coatings. Corros. Sci. 2003, 45, 181–198. [Google Scholar] [CrossRef]
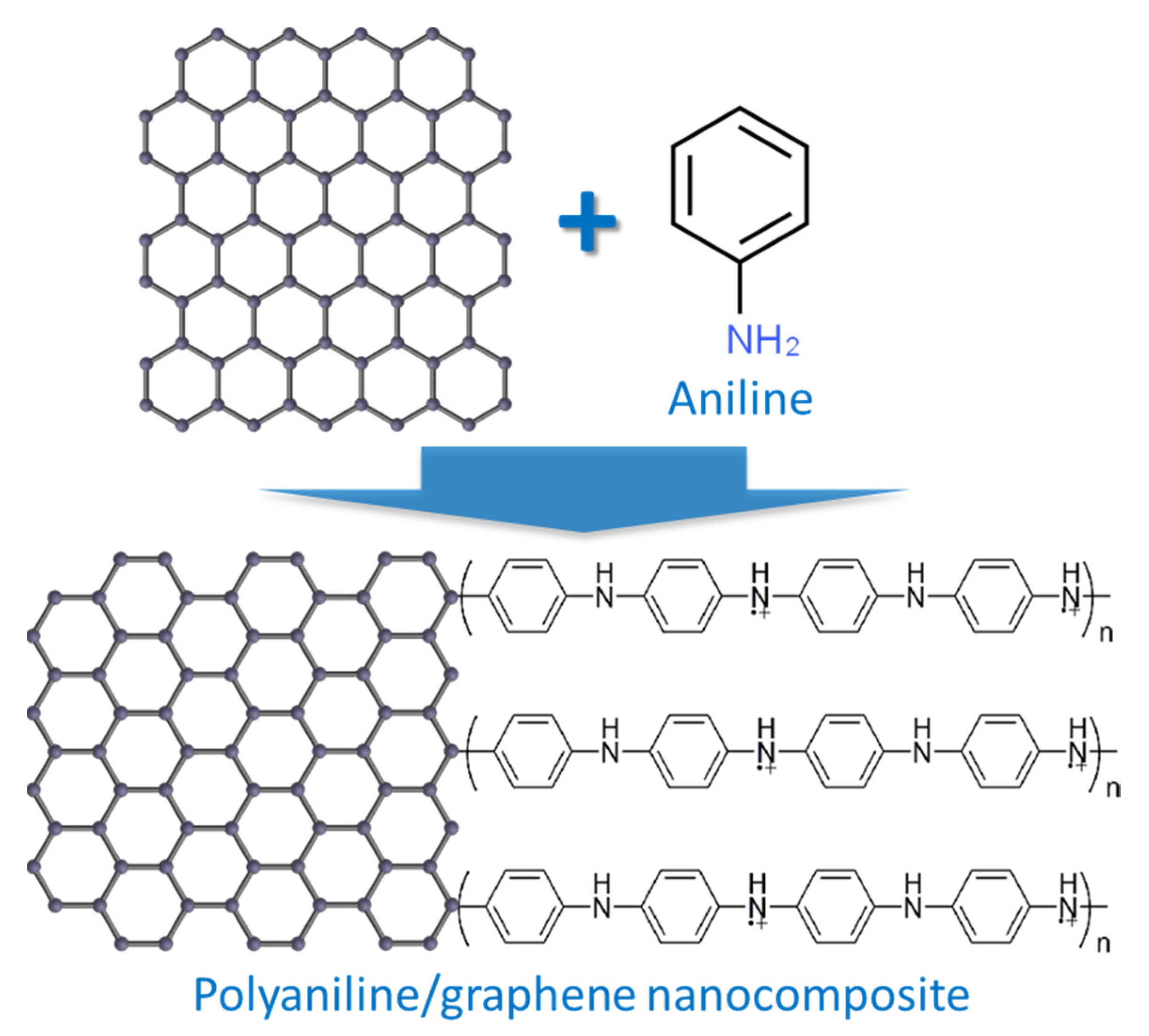
- Mello, H.J.N.P.D.; Mulato, M. Effect of aniline monomer concentration on PANI electropolymerization process and its influence for applications in chemical sensors. Synth. Met. 2018, 239, 66–70. [Google Scholar] [CrossRef]
- Zhou, Y.; She, W.; Hou, D.; Yin, B.; Chang, H.; Jiang, J.; Li, J. Modification of incorporation and in-situ polymerization of aniline on the nano-structure and meso-structure of calcium silicate hydrates. Constr. Build. Mater. 2018, 182, 459–468. [Google Scholar] [CrossRef]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-based aromatic epoxy monomers for thermoset materials. Molecules 2017, 22, 149. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, L.; Wu, T.; Pan, Y.; Liu, G. Synthesis of low-electrical-conductivity graphene/pernigraniline composites and their application in corrosion protection. Carbon 2014, 79, 605–614. [Google Scholar] [CrossRef]
- Bakir, M. Design and Characterization of Aromatic Thermosetting Copolyester Resin for Polymer Matrix Nanocomposites. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2019. [Google Scholar]
- Kausar, A. Graphene nanomesh and polymeric material at cutting edge. Polym.-Plast. Technol. Mater. 2019, 58, 803–820. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Li, M.; Ji, X.; Cui, L.; Liu, J. In situ preparation of graphene/polypyrrole nanocomposite via electrochemical co-deposition methodology for anti-corrosion application. J. Mater. Sci. 2017, 52, 12251–12265. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tsai, P.Y.; Gu, B.E.; Hu, W.C.; Jhao, J.S.; Jhuang, G.S.; Lee, Y.L. The development of novel sound-absorbing and anti-corrosion nanocomposite coating. ECS Trans. 2016, 72, 171. [Google Scholar] [CrossRef]
- Oladele, I.O.; Omotosho, T.F.; Adediran, A.A. Polymer-based composites: An indispensable material for present and future applications. Int. J. Polym. Sci. 2020, 2020, 8834518. [Google Scholar] [CrossRef]
- Adhikari, M.; Orasugh, J.T.; Chattopadhyay, D. Biomedical Application of Polymer-Graphene Composites. In Polymer Nanocomposites Containing Graphene; Elsevier: Amsterdam, The Netherlands, 2022; pp. 507–535. [Google Scholar]
- Patra, S.; Swain, S.K. Graphene-Based Nanocomposites for Biomedical Engineering Application. In Green Biocomposites for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 197–224. [Google Scholar]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, A. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Lotfi, R.; Zandi, N.; Mazaheri, M.; Soleimani, F.; Simchi, A. Graphene-Based Polymer Nanocomposites in Biomedical Applications. In Innovations in Graphene-Based Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 199–245. [Google Scholar]
- Singh, K.D.; Verma, R.K. Development of reduced Graphene oxide modified ultrahigh molecular weight polyethylene (rGO/UHMWPE) based nanocomposites for biomedical applications. J. Thermoplast. Compos. Mater. 2022, OnlineFirst. [Google Scholar] [CrossRef]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene oxide-incorporated hydrogels for biomedical applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Kausar, A. Scope of Polymer/Graphene Nanocomposite in Defense Relevance: Defense Application of Polymer/Graphene. In Polymer Nanocomposites for Advanced Engineering and Military Applications; IGI Global: Hershey, PA, USA, 2019; pp. 296–315. [Google Scholar]
- Silva, M.; Alves, N.M.; Paiva, M.C. Graphene-polymer nanocomposites for biomedical applications. Polym. Adv. Technol. 2018, 29, 687–700. [Google Scholar] [CrossRef]
- Niyobuhungiro, D.; Hong, L. Graphene polymer composites: Art of review on fabrication method, properties, and future perspectives. Adv. Sci. Technol. Res. J. 2021, 15, 37–49. [Google Scholar] [CrossRef]
- Dai, D.; Zhou, D.; Xie, H.; Wang, J.; Zhang, C. The design, construction and application of graphene family composite nanocoating on dental metal surface. Biomater. Adv. 2022, 140, 213087. [Google Scholar] [CrossRef] [PubMed]
- Zagho, M.M.; Hussein, E.A.; Elzatahry, A.A. Recent overviews in functional polymer composites for biomedical applications. Polymers 2018, 10, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokaya, D.; Srimaneepong, V.; Thunyakitpisal, P.; Qin, J.; Rosa, V.; Sapkota, J. Potential Applications of Graphene-Based Nanomaterials in Biomedical, Dental, and Implant Applications. In Advances in Dental Implantology Using Nanomaterials and Allied Technology Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 77–105. [Google Scholar]
- Cheekuramelli, N.S.; Late, D.; Kiran, S.; Garnaik, B. Biodegradable and Biocompatible Polymer Composite: Biomedical Applications and Bioimplants. In Lightweight Polymer Composite Structures; CRC Press: Boca Raton, FL, USA, 2020; pp. 67–88. [Google Scholar]
- Wen, C.; Zhan, X.; Huang, X.; Xu, F.; Luo, L.; Xia, C. Characterization and corrosion properties of hydroxyapatite/graphene oxide bio-composite coating on magnesium alloy by one-step micro-arc oxidation method. Surf. Coat. Technol. 2017, 317, 125–133. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Y.; Gao, H. Improving the corrosion resistance and osteogenic differentiation of ZK60 magnesium alloys by hydroxyapatite/graphene/graphene oxide composite coating. Ceram. Int. 2022, 48, 16131–16141. [Google Scholar] [CrossRef]
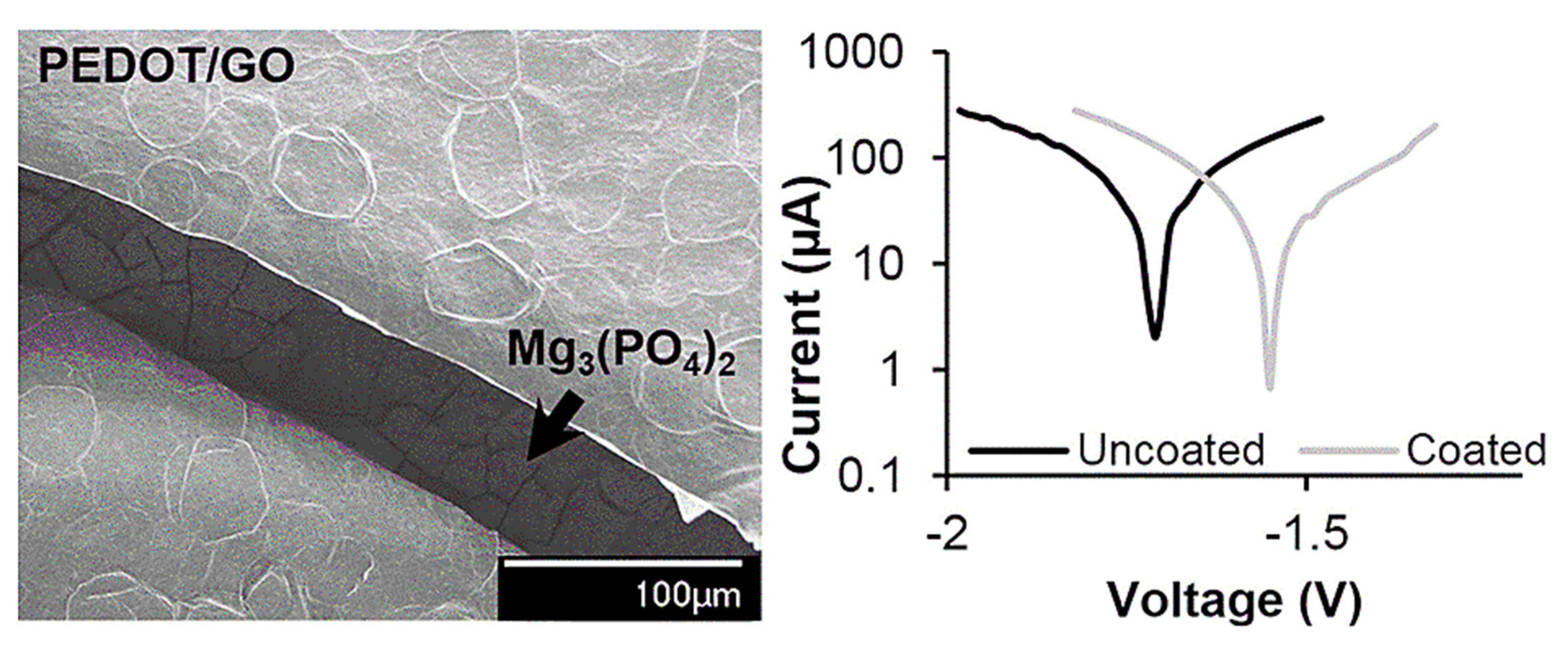
- Catt, K.; Li, H.; Cui, X.T. Poly (3, 4-ethylenedioxythiophene) graphene oxide composite coatings for controlling magnesium implant corrosion. Acta Biomater. 2017, 48, 530–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, J.; van de Kemp, T.; Costa-Almeida, R.; Pereira, R.; Magalhães, F.D.; Castilho, M.; Pinto, A.M. Fabrication of polymer/graphene biocomposites for tissue engineering. Polymers 2022, 14, 1038. [Google Scholar] [CrossRef]
- Rikhari, B.; Mani, S.P.; Rajendran, N. Polypyrrole/graphene oxide composite coating on Ti implants: A promising material for biomedical applications. J. Mater. Sci. 2020, 55, 5211–5229. [Google Scholar] [CrossRef]
- de Armentia, S.L.; Fernández-Villamarín, S.; Ballesteros, Y.; Del Real, J.; Dunne, N.; Paz, E. 3D printing of a graphene-modified photopolymer using stereolithography for biomedical applications: A study of the polymerization reaction. Int. J. Bioprint. 2022, 8, 503. [Google Scholar] [CrossRef]
- Ou, L.; Bin Song, B.; Liang, H.; Liu, J.; Feng, X.; Bin Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, L.; Kulesza, P.J. Fabrication of composite coatings of 4-(pyrrole-1-yl) benzoate-modified poly-3, 4-ethylenedioxythiophene with phosphomolybdate and their application in corrosion protection. Electrochim. Acta 2011, 56, 3649–3655. [Google Scholar] [CrossRef]
- Pavase, T.R.; Lin, H.; Shaikh, Q.-U.-A.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent advances of conjugated polymer (CP) nanocomposite-based chemical sensors and their applications in food spoilage detection: A comprehensive review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Gergely, A.; Pászti, Z.; Hakkel, O.; Drotár, E.; Mihály, J.; Kálmán, E. Corrosion protection of cold-rolled steel with alkyd paint coatings composited with submicron-structure types polypyrrole-modified nano-size alumina and carbon nanotubes. Mater. Sci. Eng. B 2012, 177, 1571–1582. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Montemor, M. “SMART” protective ability of water based epoxy coatings loaded with CaCO3 microbeads impregnated with corrosion inhibitors applied on AA2024 substrates. Electrochim. Acta 2012, 83, 439–447. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Forsgren, A. Corrosion Control through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Othman, N.H.; Ismail, M.C.; Mustapha, M.; Sallih, N.; Kee, K.E.; Jaal, R.A. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Qi, X.; Lin, Z.; Battocchi, D. Graphene reinforced composites as protective coatings for oil and gas pipelines. Nanomaterials 2018, 8, 1005. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Bocchetta, P. High-Performance Corrosion-Resistant Polymer/Graphene Nanomaterials for Biomedical Relevance. J. Compos. Sci. 2022, 6, 362. https://doi.org/10.3390/jcs6120362
Kausar A, Ahmad I, Bocchetta P. High-Performance Corrosion-Resistant Polymer/Graphene Nanomaterials for Biomedical Relevance. Journal of Composites Science. 2022; 6(12):362. https://doi.org/10.3390/jcs6120362
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, and Patrizia Bocchetta. 2022. "High-Performance Corrosion-Resistant Polymer/Graphene Nanomaterials for Biomedical Relevance" Journal of Composites Science 6, no. 12: 362. https://doi.org/10.3390/jcs6120362
APA StyleKausar, A., Ahmad, I., & Bocchetta, P. (2022). High-Performance Corrosion-Resistant Polymer/Graphene Nanomaterials for Biomedical Relevance. Journal of Composites Science, 6(12), 362. https://doi.org/10.3390/jcs6120362