Simple Approach to the Fabrication of Lanthanum Orthoniobates and Nanocomposites with Ni, Cu, and Co Metal Nanoparticles Using Supercritical Isopropanol
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Characteristics
3.1.1. Niobates
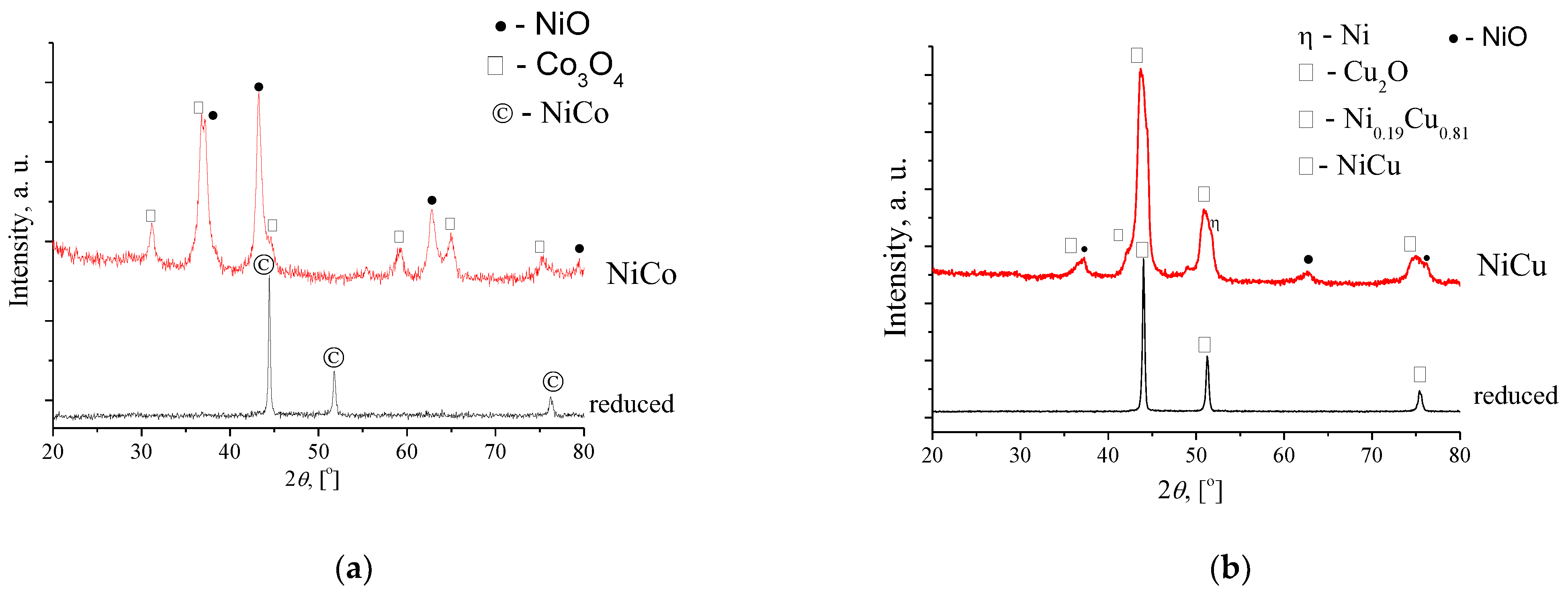
3.1.2. NiCu and NiCo
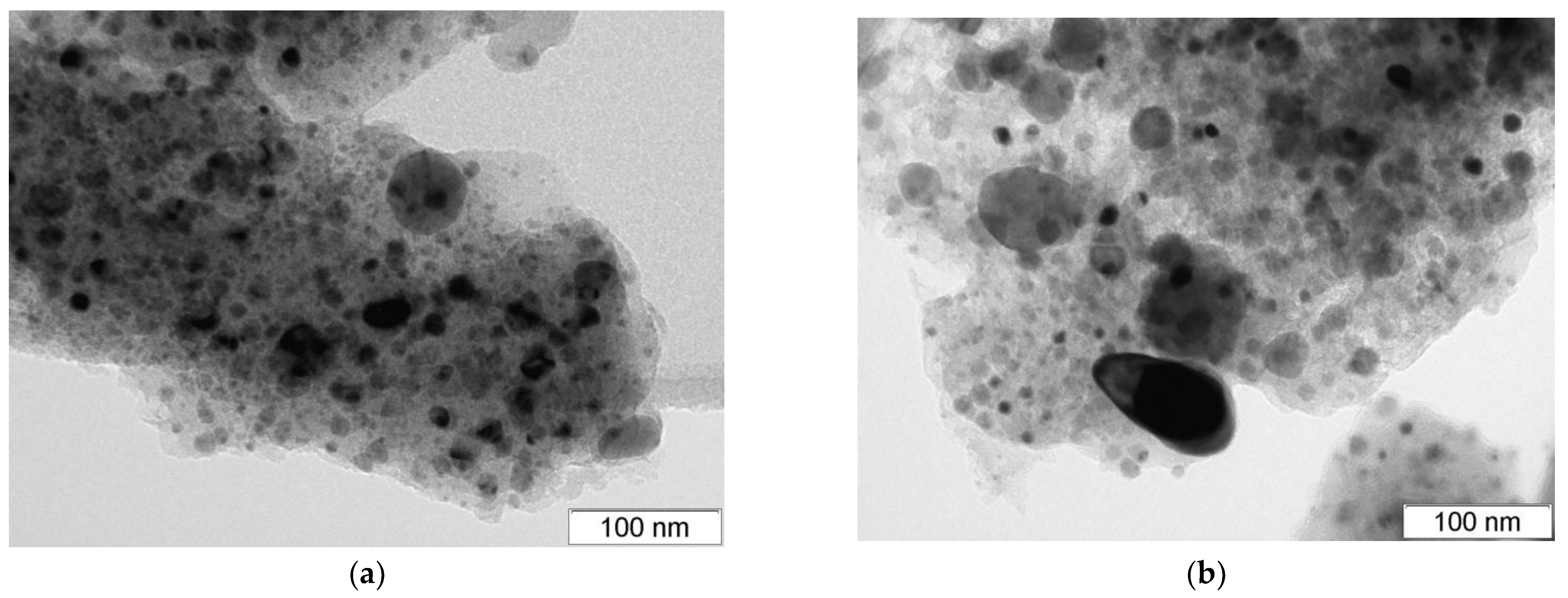
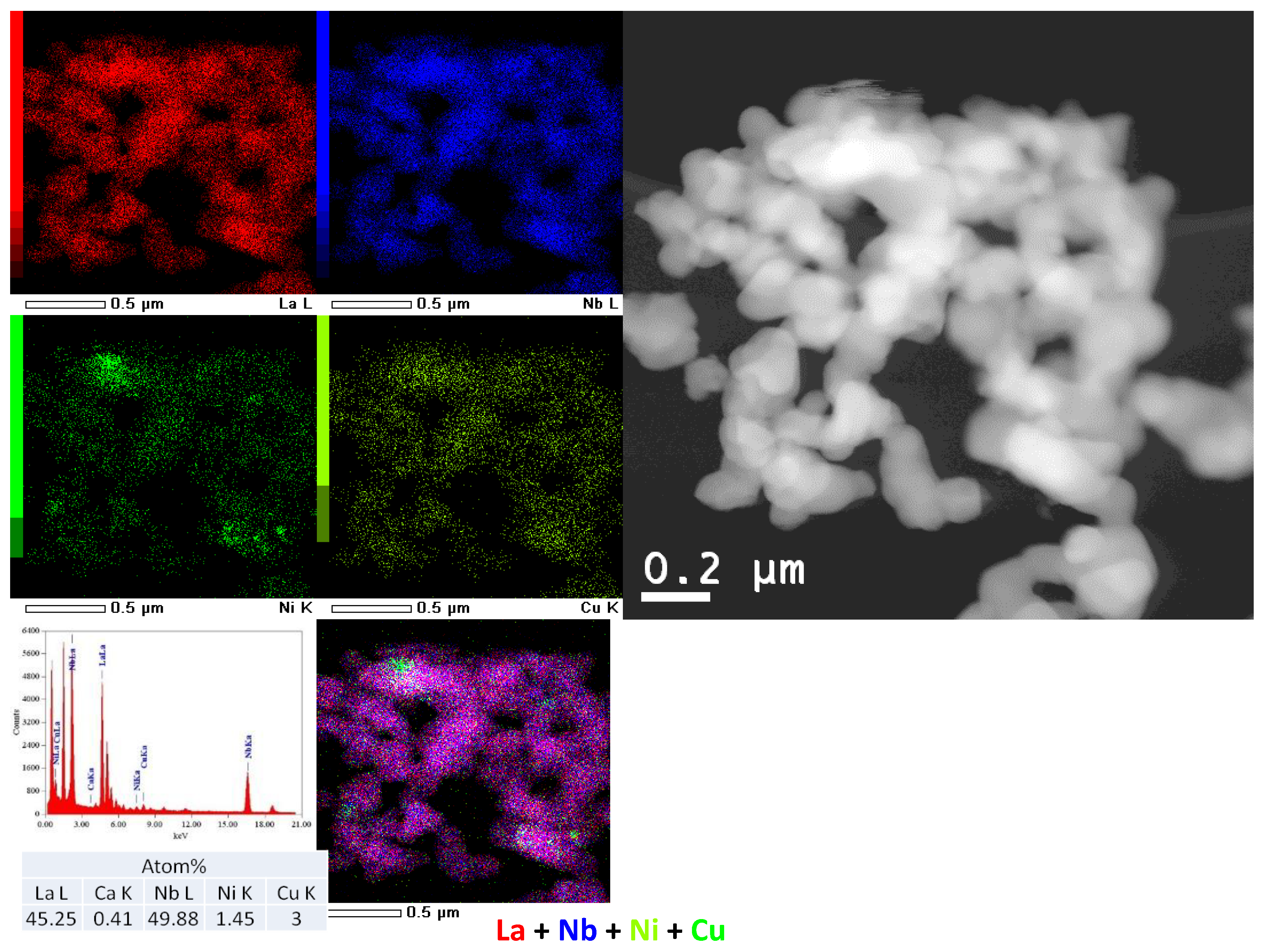
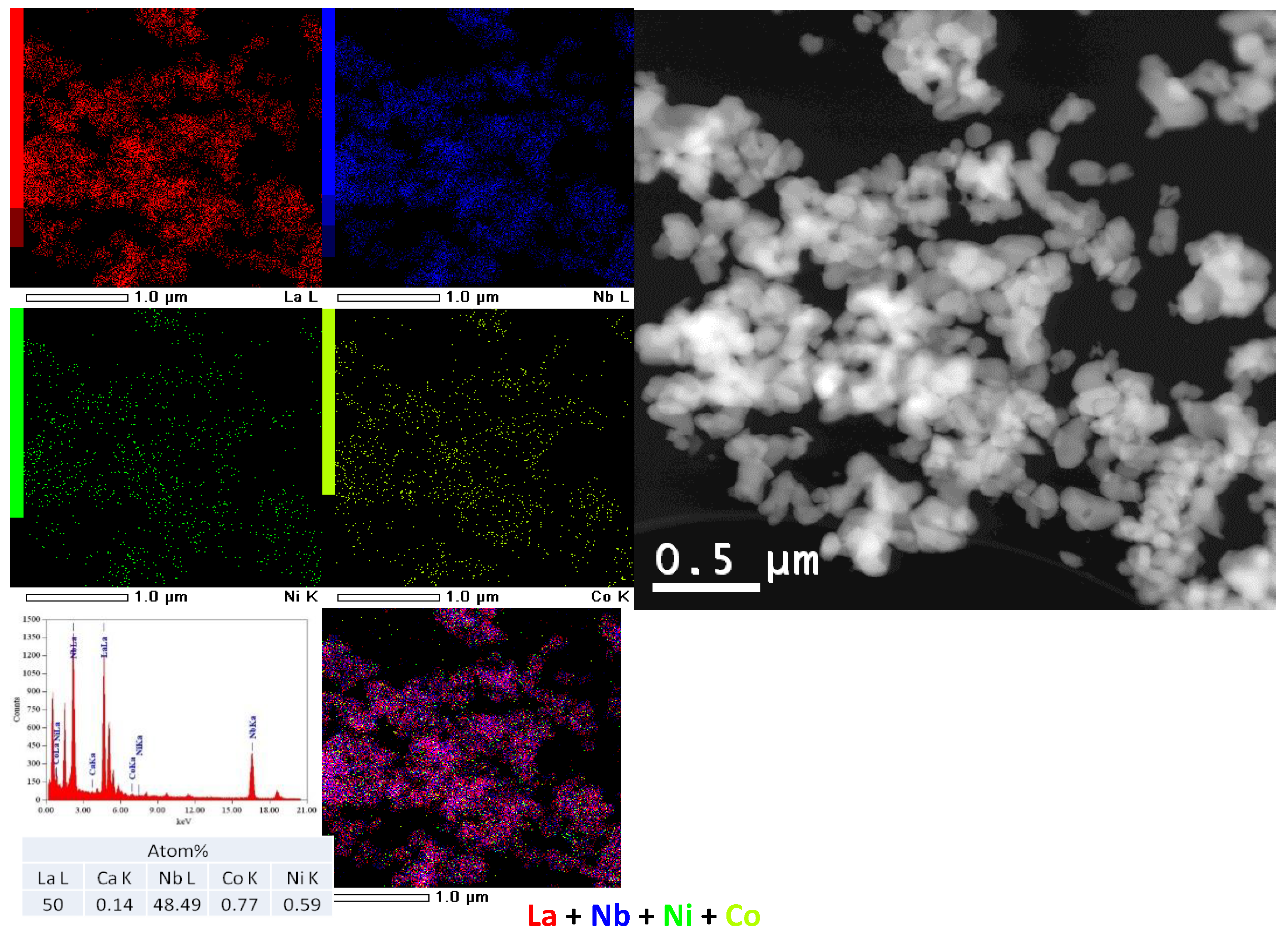
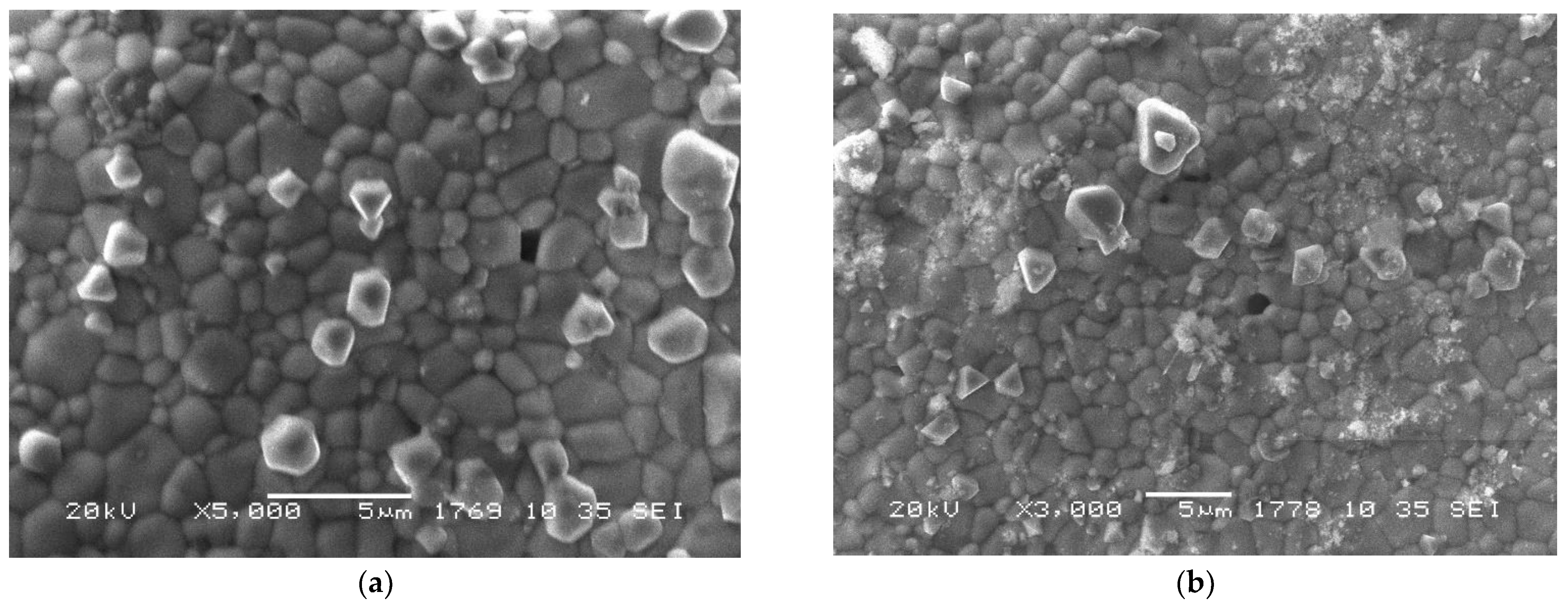
3.1.3. Nanocomposites
3.2. Reducibility in Hydrogen
3.3. Conductivity
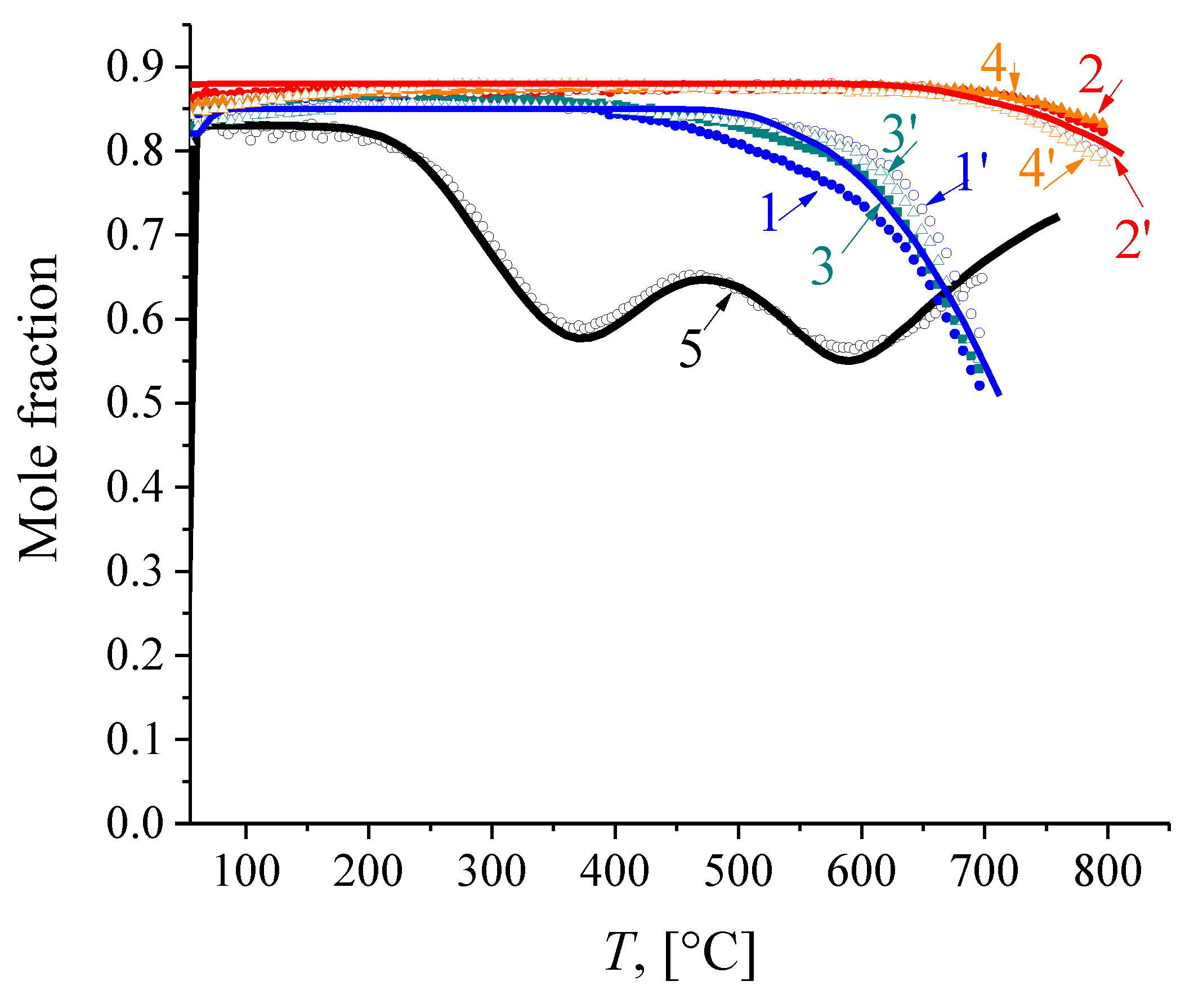
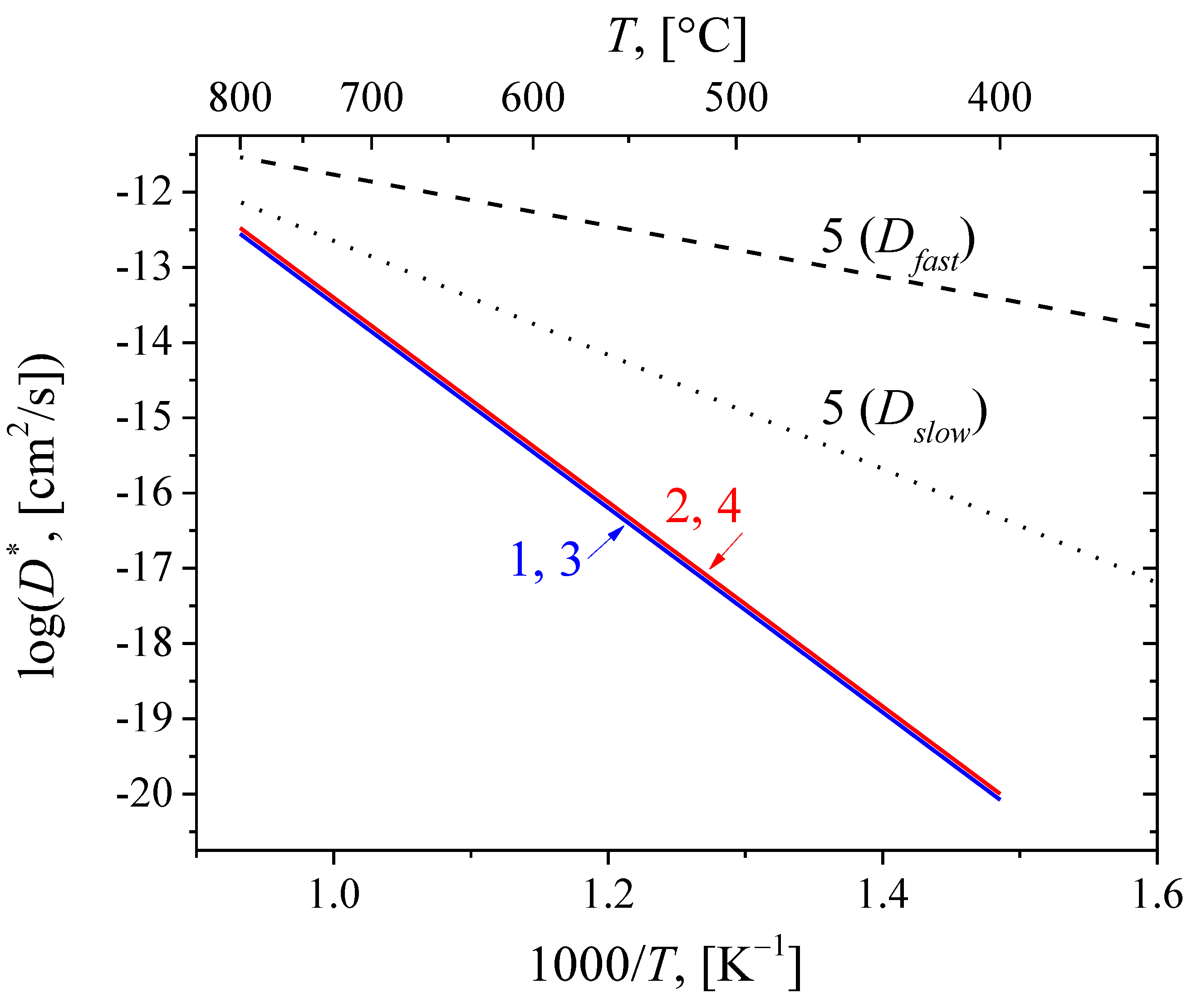
3.4. Oxygen Mobility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Sengodan, S.; Kim, S.; Kwon, O.; Bu, Y.; Kim, G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2019, 109, 606–618. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, J.; Yuan, Z.; Liu, J.; Ni, M.; Chen, F. Progress Report on Proton Conducting Solid Oxide Electrolysis Cells. Adv. Funct. Mater. 2019, 29, 1903805. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2020, 50, 1138–1187. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, V.A.; Bespalko, Y.N.; Krasnov, A.V.; Skriabin, P.I.; Lukashevich, A.I.; Fedorova, Y.E.; Sadovskaya, E.M.; Eremeev, N.F.; Krieger, T.A.; Ishchenko, A.V.; et al. Novel proton-conducting nanocomposites for hydrogen separation membranes. Solid State Ion. 2018, 322, 69–78. [Google Scholar] [CrossRef]
- Solís, C.; Serra, J.M. Adjusting the conduction properties of La0.995Ca 0.005NbO4-δ by doping for proton conducting fuel cells electrode operation. Solid State Ion. 2011, 190, 38–45. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Harris, C.M.; Skinner, S.J. Redox behaviour and solid solubility of cerium ortho-niobates. J. Solid State Chem. 2019, 271, 135–143. [Google Scholar] [CrossRef]
- Magrasó, A.; Haugsrud, R.; Norby, T. Preparation and characterization of Ni-LaNbO4 cermet anode supports for proton-conducting fuel cell applications. J. Am. Ceram. Soc. 2010, 93, 2650–2655. [Google Scholar] [CrossRef]
- Chiara, A.; Canu, G.; Longo, A.; Pipitone, C.; Martorana, A.; Giannici, F. Solid-state compatibility of Ca:LaNbO4 with perovskite cathodes: Evidences from X-ray microspectroscopy. Electrochim. Acta 2022, 401, 139495. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Norby, T.; Haugsrud, R. Dense Ceramic Membranes for Hydrogen Separation; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; ISBN 3527313427. [Google Scholar]
- Mokkelbost, T.; Lein, H.L.; Vullum, P.E.; Holmestad, R.; Grande, T.; Einarsrud, M.A. Thermal and mechanical properties of LaNbO4-based ceramics. Ceram. Int. 2009, 35, 2877–2883. [Google Scholar] [CrossRef]
- Cao, Y.; Duan, N.; Yan, D.; Chi, B.; Pu, J.; Jian, L. Enhanced electrical conductivity of LaNbO4 by A-site substitution. Int. J. Hydrog. Energy 2016, 41, 20633–20639. [Google Scholar] [CrossRef]
- Syvertsen, G.E.; Magrasó, A.; Haugsrud, R.; Einarsrud, M.A.; Grande, T. The effect of cation non-stoichiometry in LaNbO4 materials. Int. J. Hydrog. Energy 2012, 37, 8017–8026. [Google Scholar] [CrossRef]
- Ivanova, M.; Ricote, S.; Meulenberg, W.A.; Haugsrud, R.; Ziegner, M. Effects of A- and B-site (co-)acceptor doping on the structure and proton conductivity of LaNbO4. Solid State Ion. 2012, 213, 45–52. [Google Scholar] [CrossRef]
- Dzierzgowski, K.; Wachowski, S.; Gojtowska, W.; Lewandowska, I.; Jasiński, P.; Gazda, M.; Mielewczyk-Gryń, A. Praseodymium substituted lanthanum orthoniobate: Electrical and structural properties. Ceram. Int. 2018, 44, 8210–8215. [Google Scholar] [CrossRef]
- Fjeld, H.; Kepaptsoglou, D.M.; Haugsrud, R.; Norby, T. Charge carriers in grain boundaries of 0.5% Sr-doped LaNbO4. Solid State Ion. 2010, 181, 104–109. [Google Scholar] [CrossRef]
- Cao, Y.; Chi, B.; Pu, J.; Jian, L. Effect of Ce and Yb co-doping on conductivity of LaNbO4. J. Eur. Ceram. Soc. 2014, 34, 1981–1988. [Google Scholar] [CrossRef]
- Hakimova, L.; Kasyanova, A.; Farlenkov, A.; Lyagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Effect of isovalent substitution of La3+ in Ca-doped LaNbO4 on the thermal and electrical properties. Ceram. Int. 2019, 45, 209–215. [Google Scholar] [CrossRef]
- Dzierzgowski, K.; Wachowski, S.; Łapiński, M.; Mielewczyk-Gryń, A.; Gazda, M. Praseodymium Orthoniobate and Praseodymium Substituted Lanthanum Orthoniobate: Electrical and Structural Properties. Materials 2022, 15, 2267. [Google Scholar] [CrossRef]
- Canu, G.; Giannici, F.; Chiara, A.; Confalonieri, G.; Longo, A.; Teresa, M.; Dapiaggi, M.; Buscaglia, V.; Martorana, A. combined synchrotron techniques: Structure, W oxidation state and interdiffusion. J. Alloys Compd. 2021, 857, 157532. [Google Scholar] [CrossRef]
- Li, M.; Wu, R.; Zhu, L.; Cheng, J.; Hong, T.; Xu, C. Enhanced sinterability and conductivity of cobalt doped lanthanum niobate as electrolyte for proton-conducting solid oxide fuel cell. Ceram. Int. 2019, 45, 573–578. [Google Scholar] [CrossRef]
- Brandão, A.D.; Gracio, J.; Mather, G.C.; Kharton, V.V.; Fagg, D.P. B-site substitutions in LaNb1-xMxO4-δ materials in the search for potential proton conductors (M=Ga, Ge, Si, B, Ti, Zr, P, Al). J. Solid State Chem. 2011, 184, 863–870. [Google Scholar] [CrossRef]
- Cao, Y.; Duan, N.; Wang, X.; Chi, B.; Pu, J.; Jian, L. Enhanced electrical conductivity of Mo-doped LaNbO4. J. Eur. Ceram. Soc. 2015, 35, 1979–1983. [Google Scholar] [CrossRef]
- Sayers, R.; Kilner, J.A.; Skinner, S.J. Investigation of Ruddlesden-Popper Based Materials for Cathode Applications in Intermediate Temperature Sofcs; Imperial College London: London, UK, 2008; Volume 135, p. 2725. [Google Scholar]
- Li, C.; Bayliss, R.D.; Skinner, S.J. Crystal structure and potential interstitial oxide ion conductivity of LnNbO4 and LnNb0.92W0.08O4.04 (Ln = La, Pr, Nd). Solid State Ion. 2014, 262, 530–535. [Google Scholar] [CrossRef]
- Bi, Z.; Bridges, C.A.; Kim, J.H.; Huq, A.; Paranthaman, M.P. Phase stability and electrical conductivity of Ca-doped LaNb1-xTaxO4-δ high temperature proton conductors. J. Power Sources 2011, 196, 7395–7403. [Google Scholar] [CrossRef]
- Huse, M.; Norby, T.; Haugsrud, R. Effects of A and B site acceptor doping on hydration and proton mobility of LaNbO4. Int. J. Hydrog. Energy 2012, 37, 8004–8016. [Google Scholar] [CrossRef]
- Magrasó, A.; Fontaine, M.L. Investigation of compatible anode systems for LaNbO4-based electrolyte in novel proton conducting solid oxide fuel cells. J. Power Sources 2011, 196, 10183–10190. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, J.H.; Nahm, S.; Kim, D.W.; Park, J.G. Low-temperature sintering of temperature-stable LaNbO4 microwave dielectric ceramics. Mater. Res. Bull. 2010, 45, 21–24. [Google Scholar] [CrossRef]
- Maschio, S.; Bachiorrini, A.; Di Monte, R.; Montanaro, L. Preparation and characterization of LaNbO4 from amorphous precursors. J. Mater. Sci. 1995, 30, 5433–5437. [Google Scholar] [CrossRef]
- Mielewczyk-Gryn, A.; Wachowski, S.; Zagórski, K.; Jasiński, P.; Gazda, M. Characterization of magnesium doped lanthanum orthoniobate synthesized by molten salt route. Ceram. Int. 2015, 41, 7847–7852. [Google Scholar] [CrossRef]
- Bruncková, H.; Medvecký, L.; Hvizdoš, P.; Ďurišin, J.; Girman, V. Structural and mechanical properties of sol–gel prepared pyrochlore lanthanum niobates. J. Mater. Sci. 2015, 50, 7197–7207. [Google Scholar] [CrossRef]
- Mokkelbost, T.; Kaus, I.; Haugsrud, R.; Norby, T.; Grande, T.; Einarsrud, M.A. High-temperature proton-conducting lanthanum ortho-niobate-based materials. Part II: Sintering properties and solubility of alkaline earth oxides. J. Am. Ceram. Soc. 2008, 91, 879–886. [Google Scholar] [CrossRef]
- Aymonier, C.; Loppinet-Serani, A.; Reverón, H.; Garrabos, Y.; Cansell, F. Review of supercritical fluids in inorganic materials science. J. Supercrit. Fluids 2006, 38, 242–251. [Google Scholar] [CrossRef]
- Toyama, S.; Hayashi, H.; Takesue, M.; Watanabe, M.; Smith, R.L. Synthesis of alkali niobate K1-xNaxNbO3 nanoparticles using a supercritical water flow system. J. Supercrit. Fluids 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R. Nanomaterials and supercritical fluids. J. Supercrit. Fluids 2006, 37, 1–22. [Google Scholar] [CrossRef]
- Ivicheva, S.N.; Kargin, Y.F.; Gorelik, V.S. Opal-matrix nanocomposites containing metallic nanoparticles. Inorg. Mater. 2015, 51, 840–847. [Google Scholar] [CrossRef]
- Choi, H.; Veriansyah, B.; Kim, J.; Kim, J.D.; Kang, J.W. Continuous synthesis of metal nanoparticles in supercritical methanol. J. Supercrit. Fluids 2010, 52, 285–291. [Google Scholar] [CrossRef]
- Altynbekova, D.; Massalimova, B.; Bespalko, Y.; Valeev, K.; Sadykov, V. Synthesis of Metal Nanoparticles and Their Alloys Using Supercritical Fluids; Her. Kazakh-British Technical University: Almaty, Kazakhstan, 2020; p. 17. [Google Scholar]
- Bespalko, Y.; Smal, E.; Simonov, M.; Valeev, K.; Fedorova, V. Novel Ni/Ce(Ti) ZrO2 catalysts for methane dry reforming prepared in supercritical alcohol media. Energies 2020, 13, 3365. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Ulihin, A.S.; Bespalko, Y.N.; Eremeev, N.F.; Krasnov, A.V.; Skriabin, P.I.; Sadykov, V.A. Study of proton conductivity of composite metal-ceramic materials based on neodimium tugstates using a four-electrode technique with ionic probes. Int. J. Hydrog. Energy 2018, 43, 19521–19527. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Eremeev, N.F.; Skriabin, P.I.; Krasnov, A.V.; Bespalko, Y.N.; Pavlova, S.N.; Fedorova, Y.E.; Pikalova, E.Y.; Shlyakhtina, A.V. Oxygen Mobility in the Materials for Solid Oxide Fuel Cells and Catalytic Membranes (Review). Russ. J. Electrochem. 2019, 55, 701–718. [Google Scholar] [CrossRef]
- Xing, W.; Syvertsen, G.E.; Grande, T.; Li, Z.; Haugsrud, R. Hydrogen permeation, transport properties and microstructure of Ca-doped LaNbO4 and LaNb3O9 composites. J. Membr. Sci. 2012, 415, 878–885. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni supported on CeO2 as selective bimetallic catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Bespalko, Y.; Sadykov, V.; Eremeev, N.; Skryabin, P.; Krieger, T.; Sadovskaya, E.; Bobrova, L.; Uvarov, N.; Lukashevich, A.; Krasnov, A.; et al. Synthesis of tungstates/Ni0.5Cu0.5O nanocomposite materials for hydrogen separation cermet membranes. Compos. Struct. 2018, 202, 1263–1274. [Google Scholar] [CrossRef]
- Haugsrud, R.; Norby, T. High-temperature proton conductivity in acceptor-doped LaNbO4. Solid State Ion. 2006, 177, 1129–1135. [Google Scholar] [CrossRef]



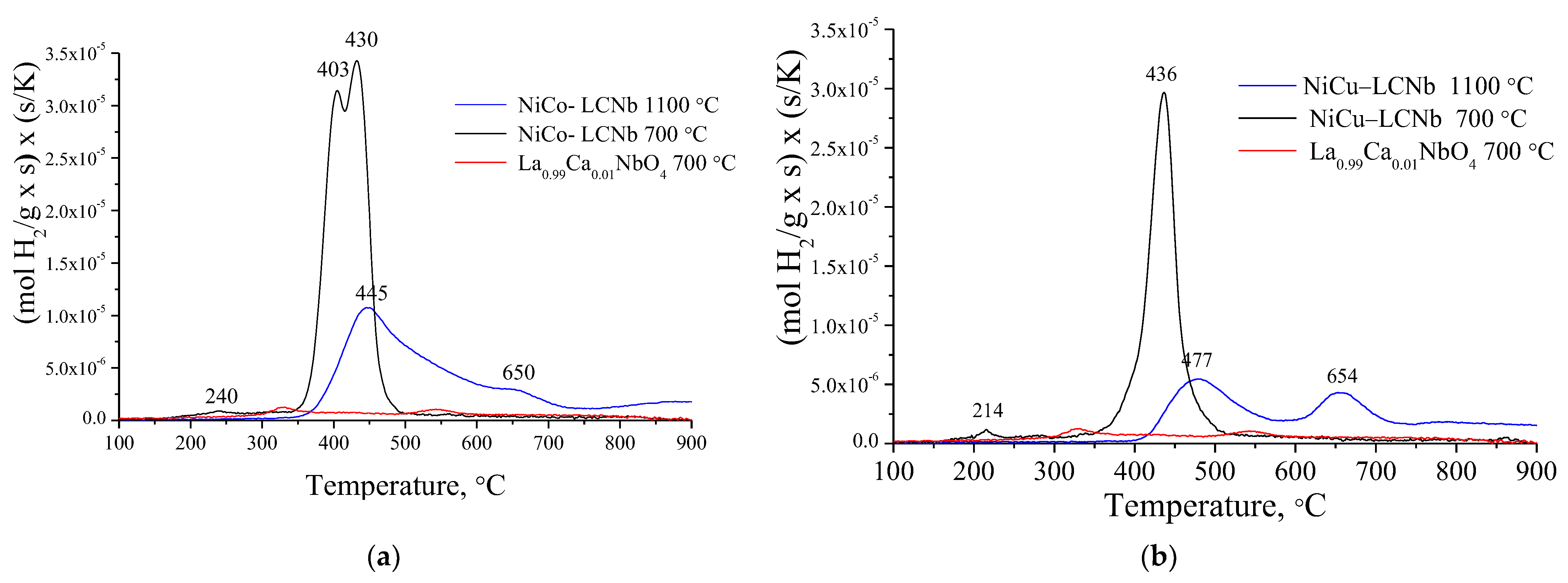


| № | Code | Composition | Relative Density, % | SSA, m2/g | |
|---|---|---|---|---|---|
| 700 °C | 1100 °C | ||||
| 1 | LCNb | La0.99Ca0.01NbO4 | 77 | 13 | 1.5 |
| 2 | LCNbTi | La0.99Ca0.01Nb0.98Ti0.02O4–δ | 74 | 14.6 | 5.9 |
| 3 | NiCu | Ni0.5Cu0.5Ox | - | 8 * | - |
| 4 | NiCo | Ni0.5Co0.5Ox | - | 10 * | - |
| 5 | NiCu–LCNb | La0.99Ca0.01NbO4/NiCuOx | 82 | 4.5 | 0.3 |
| 6 | NiCo–LCNb | La0.99Ca0.01NbO4/NiCoOx | 85 | 5.3 | 0.1 |
| № | Sample | Tcalcination, °C | The Maxima of Peaks, °C | H2 Consumption, mol H2 g−1 × 10−3 | |
|---|---|---|---|---|---|
| T1 | T2 | ||||
| 1 | LCNb | 700 | 325 | 544 | 0.4 |
| 2 | NiCu–LCNb | 700 | 436 | - | 1.8 |
| 1100 | 477 | 654 | 1.3 | ||
| 3 | NiCo–LCNb | 700 | 403 | 430 | 2.8 |
| 1100 | 445 | - | 2.1 | ||
| Sample | Tcalcination, °C | D* |700 K, [cm2/s] | Ea,D, [kJ/mol] |
|---|---|---|---|
| NiCu–LCNb | 700 | 5(±3)∙10–20 | |
| 1100 | 6(±4)∙10–20 | ||
| NiCo–LCNb | 700 | 5(±3)∙10–20 | 260 |
| 1100 | 6(±4)∙10–20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altynbekova, D.; Bespalko, Y.; Valeev, K.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Ulihin, A.; Uhina, A.; Massalimova, B.; Simonov, M.; et al. Simple Approach to the Fabrication of Lanthanum Orthoniobates and Nanocomposites with Ni, Cu, and Co Metal Nanoparticles Using Supercritical Isopropanol. J. Compos. Sci. 2022, 6, 243. https://doi.org/10.3390/jcs6090243
Altynbekova D, Bespalko Y, Valeev K, Eremeev N, Sadovskaya E, Krieger T, Ulihin A, Uhina A, Massalimova B, Simonov M, et al. Simple Approach to the Fabrication of Lanthanum Orthoniobates and Nanocomposites with Ni, Cu, and Co Metal Nanoparticles Using Supercritical Isopropanol. Journal of Composites Science. 2022; 6(9):243. https://doi.org/10.3390/jcs6090243
Chicago/Turabian StyleAltynbekova, Dinara, Yuliya Bespalko, Konstantin Valeev, Nikita Eremeev, Ekaterina Sadovskaya, Tamara Krieger, Artem Ulihin, Arina Uhina, Bakytgul Massalimova, Mikhail Simonov, and et al. 2022. "Simple Approach to the Fabrication of Lanthanum Orthoniobates and Nanocomposites with Ni, Cu, and Co Metal Nanoparticles Using Supercritical Isopropanol" Journal of Composites Science 6, no. 9: 243. https://doi.org/10.3390/jcs6090243
APA StyleAltynbekova, D., Bespalko, Y., Valeev, K., Eremeev, N., Sadovskaya, E., Krieger, T., Ulihin, A., Uhina, A., Massalimova, B., Simonov, M., & Sadykov, V. (2022). Simple Approach to the Fabrication of Lanthanum Orthoniobates and Nanocomposites with Ni, Cu, and Co Metal Nanoparticles Using Supercritical Isopropanol. Journal of Composites Science, 6(9), 243. https://doi.org/10.3390/jcs6090243








