1. Introduction
Currently, there are many works focused on using accumulated anthropogenic waste from mining and metallurgical enterprises, such as overburden, beneficiation waste, slag, in processing [
1,
2,
3]. This will enable the additional extraction of large quantities of valuable elements, and will reduce the environmental load in specific regions. Low-quality raw materials are used in processing due to the depletion of reserves [
4]. This results in a significant increase in the amounts of raw materials subjected to crushing and abrasion, and leads to the accelerated wear of crushing and grinding equipment. As such, it is important to improve the performance characteristics of the materials used in this equipment.
White high-chromium cast irons are widely used in the production of castings subject to abrasive and impact–abrasive wear under the effect of aggressives media and at elevated temperatures. The high wear resistance of these cast irons is endowed by the presence of the carbides Me
7C
3 and Me
23C
6, which are formed when there is a high chromium content, as well as via the significant addition of alloying elements (manganese, vanadium, molybdenum, etc.). High-chromium cast irons with pre-eutectic and eutectic composition are the most widely used type in industry. These cast irons have great advantages when used under operating conditions where corrosive effects are not significant [
5].
Recently, more attention has been paid to the study of the structure and properties of hypereutectic high-chromium cast irons (HHCCI) and to the improvement of the characteristics of castings derived from these cast irons [
5,
6]. The structures of castings from such cast irons are formed primarily of hexagonal carbides (Cr, Fe)
7C
3 and eutectic γ-Fe + (Cr, Fe)
7C
3. The prospects for the use of such cast irons are generally considered high due to the fact that they contain a large proportion of carbides and are more corrosion-resistant. According to the studies of Pourasiabi H. et al. [
7], the presence of both large and small carbides in cast irons of hypereutectic composition enables them to effectively resist wear when exposed to large and small abrasive particles. The results of the studies conducted by Guo, Q. et al. [
8] indicate an increase in the wear resistance of HHCCI with an increase in the carbon content from 3.5 wt. % to 5.0 wt. %, an increase of 85.7%. The work [
9] examines the influence of carbon content (3.73; 4.21; 4.85 wt. %) on the size and morphology of primary carbides (Cr, Fe)
7C
3 when fusing a layer of cast iron on the surface of a steel plate. The authors of this work showed that with an increasing carbon concentration, the hardness of the deposited layer increases from 58 to 62 HRC, which is associated with an increase in the proportion of eutectic carbides, a decrease in their size, and a change in their morphology from plate-like to rod-shaped.
Studies conducted by Chang et al. [
10] have shown that when the carbon content in HHCCI increases from 3.5 wt. % to 4.85 wt. %, the corrosion resistance increases almost 20 times. At the same time, cast irons of hypoeutectic composition with increased carbon concentration (to 2.78 wt. %) are, on the contrary, characterized by reduced corrosion resistance [
11].
Previous studies have indicated that a reduction in the carbide size in the structure of HHCCI castings results in improved corrosion resistance [
6]. Abrasive abrasion resistance, strength, and crack resistance are determined by the amount of carbide phase, the size and shape of primary and eutectic carbides, their orientation, and the phase composition of the matrix [
12,
13,
14]. Cast irons with large crystalline primary carbides are prone to cracking [
15,
16].
An analysis of data from the literature indicated that a reduction in the size of primary carbides can be achieved by modifying the melt with small additions of carbide-forming (Ti, V, W, Nb, Mo) and non-carbide-forming (Y, Si, REE) elements, as well as by introducing refractory compounds that perform the role of inoculants. Meanwhile, it is not entirely clear how significant the reduction in the size of primary carbides is in the formation of the properties of HHCCI castings after their modification, since the actual reduction in primary carbide content is not significant. Introducing powders of compounds TiC [
17], TiBAl [
18] and La
2O
3 [
19], and mixtures of La
2O
3 and Ce
2O
3 [
20], leads to a reduction in the size of primary carbides by no more than 60%. The modification of primary carbides (Cr, Fe)
7C
3 by the lower and middle alloying of carbide-forming elements Ti [
21,
22], Nb [
23,
24], V [
25], W [
26,
27], and others, including with complex alloying [
25,
28], leads to a reduction in the size of primary carbides by tens of percentage points. However, with such a modification, the eutectic point actually moves to a region at a higher level of the head, i.e., the fraction of carbide (Cr, Fe)
7C
3 is removed, and the chromium concentration in austenite increases. This is due to the fact that the protective carbide-forming elements contain independent carbides. For this reason, assessing the effectiveness of this method on the structure and properties of castings made of high-chromium cast irons with a hypereutectic composition is very difficult.
According to the studies described in the work [
29], silicon affects the morphology and size of primary carbides, but its additions cause the formation of ferrite, which reduces the wear resistance of such cast irons. Additions of Ce [
30], Y, and Nd [
31] lead to a decrease (by 60%) in the size of primary carbides in HHCCI, but this result is not stable, and under certain conditions, the effects are reversed.
It is possible to influence the melt with HHCCI via thermal and temporary treatments when it is in the liquid and solid-liquid states. Thus, [
32] presents data indicating a decrease in the size of primary carbides caused by overheating the melt to above 1500 °C or by the heat treatment of a pre-melted alloy in the range of 1260–1320 °C. At the same time, the reductions in the cross-section of primary carbides in HHCCI castings reached 27–50% and 70–80%, respectively. However, these studies did not elucidate the direct influence of the sizes of primary carbides on the mechanical properties of castings.
The achievement of a large degree of melt supercooling can increase the number of crystallization centers and slow the growth of carbides, thereby reducing their size [
33,
34,
35,
36]. Melt supercooling can be achieved by casting in a metal mold, or in sand casting molds with a refrigerator. Another more promising way to reduce the size of primary crystals is to alter the crystallizing alloy by creating oscillations within it [
37], by the creation of conditions amenable to the local supercooling and formation of crystallization centers, or by suspension casting [
38].
Meanwhile, the analysis of data from the literature indicates that there are few studies on the effects of carbon content and cooling rate during the crystallization interval on the properties of HHCCI castings. The results regarding the influence of carbon content in HHCCI [
8,
9] cannot be used to predict the structure and properties of sand castings. The issue of the influence of cooling rate is indirectly considered in [
36,
39], in which samples of castings with a gradient structure were obtained using refrigerators (cooling plates or cooled crystallizers). As part of these experiments, a cooling thermogram was recorded at different points of the resulting casting. However, the authors here did not indicate the average cooling rate in the crystallization range, which complicates interpretation. The experimental results described in these publications are presented for alloys with one carbon concentration. The effect of cooling rate on the mechanical properties of castings has not been considered.
Under industrial conditions, castings from HHCCI are produced in sand molds, both with and without the use of refrigerators. Depending on the size of the castings, their cooling rates under such heat removal conditions vary significantly. Considering that the carbon content in HHCCI determines the amount of carbide phase, it becomes important to assess the influence of carbon content and cooling rate on the structure of such cast iron in the cast state and after heat treatment. The relevance of these studies is also determined by the fact that they show the actual contribution of reductions in the size of primary carbides to the formation of the properties of HHCCI castings. This therefore helps us in interpreting the mechanism of changes in the properties of castings when their structures are changed by introducing modifying additives during the smelting of such cast irons.
In this regard, the purpose of this work was to establish the effect of carbon content on the structure and mechanical properties of castings from alloy G-X300CrMo27-1 obtained at cooling rates characteristic of castings in sand molds and metal molds, both under the cast state and after destabilizing annealing.
2. Materials and Methods
Experiments designed to reveal the effects of carbon content on the structure and properties of cast iron castings were performed with alloy G-X300CrMo27-1, which is widely used in the industry. The carbon content therein varied from 2.85 to 4.5 wt. % (
Table 1), thus producing eutectic and hypereutectic alloys. Two series of experiments were conducted to assess the effects of cooling rate in the interval of crystallization of alloys on their structure and properties. The cooling rate was 0.08–0.11 °C/s in the first series of experiments, and 4.6–5 °C/s in the second one.
Alloys with different carbon contents were pre-melted in a vacuum induction furnace (UIPV-0.001) with an inductor lined with Insetag-86XT for the experiments. The charge was composed of the following materials: grade C steel, ferrochrome FeCr60C70LSLP, ferromanganese FeMn75C80VLP (A), graphite powder (0.5–2 mm), and Mo and Ni chips. Melting was conducted in a vacuum (1–4 ∗ 10−2 mm·Hg) until the complete melting of the charge components, and subsequently holding the melt at 1450–1500 °C for 30 min. Melt casting was conducted in a steel split liner with a cylindrical hole of ∅30 mm. The compositions of the castings were determined with the help of an Axios X-ray fluorescence wave dispersive combined spectrometer. The sulfur and carbon contents were determined on a Bruker G4 ICARUS TF analyzer.
In the first series of experiments, the pre-melted alloy was reheated in a tubular high-temperature furnace RHTV 120-600/C40 “Nabertherm” up to 1450 °C. The temperature was monitored with a type b thermocouple located in the vicinity of the sample. Alloy samples of 190–200 g were melted in corundum crucibles. Heating was conducted at a rate of 10 °C/min with the constant purging of the furnace with argon. After isothermal holding for 60 min to dissolve carbides, the samples were cooled together in the furnace at a rate of 0.08–0.11 °C/s. The obtained ingots were removed from the crucibles.
In the second series of experiments, the pre-melted alloy was remelted in an induction furnace. Similar to the first series of experiments, heating was conducted up to 1440–1470 °C at a residual pressure of 1–4 ∗ 10−2 mm·Hg. After holding the melt for 30 min, it was poured into a graphite liner and an ingot of ∅24 mm and 300 mm long was obtained. In this case the cooling rate in the crystallization temperature region was 4.6–5 °C/s.
The obtained specimens were cut along the vertical axis, and the sections were prepared for structural, hardness, microhardness and microprobe analyses. Castings were cut from the second half of the plate for abrasive wear tests.
The samples obtained under similar conditions were subjected to heat treatment: destabilization (or critical) heat treatments (heating at 1000 °C for 2 h) and subcritical heat treatments (500 °C for 6 h). The regimes of these heat treatments were those adopted in [
40,
41,
42]. This type of treatment is required for the decomposition of residual austenite.
The structure was studied using a Leica DM IRM inverted optical microscope. Etching was done using the reagent 100 mL HCl + 7 mL H
2SO
4 + 30 g CuSO
4 + 200 mL H
2O by rubbing with a cotton swab. The average areas and sizes of primary carbide crystals, and the fractions of primary and eutectic carbides, were assessed using the ImageJ program [
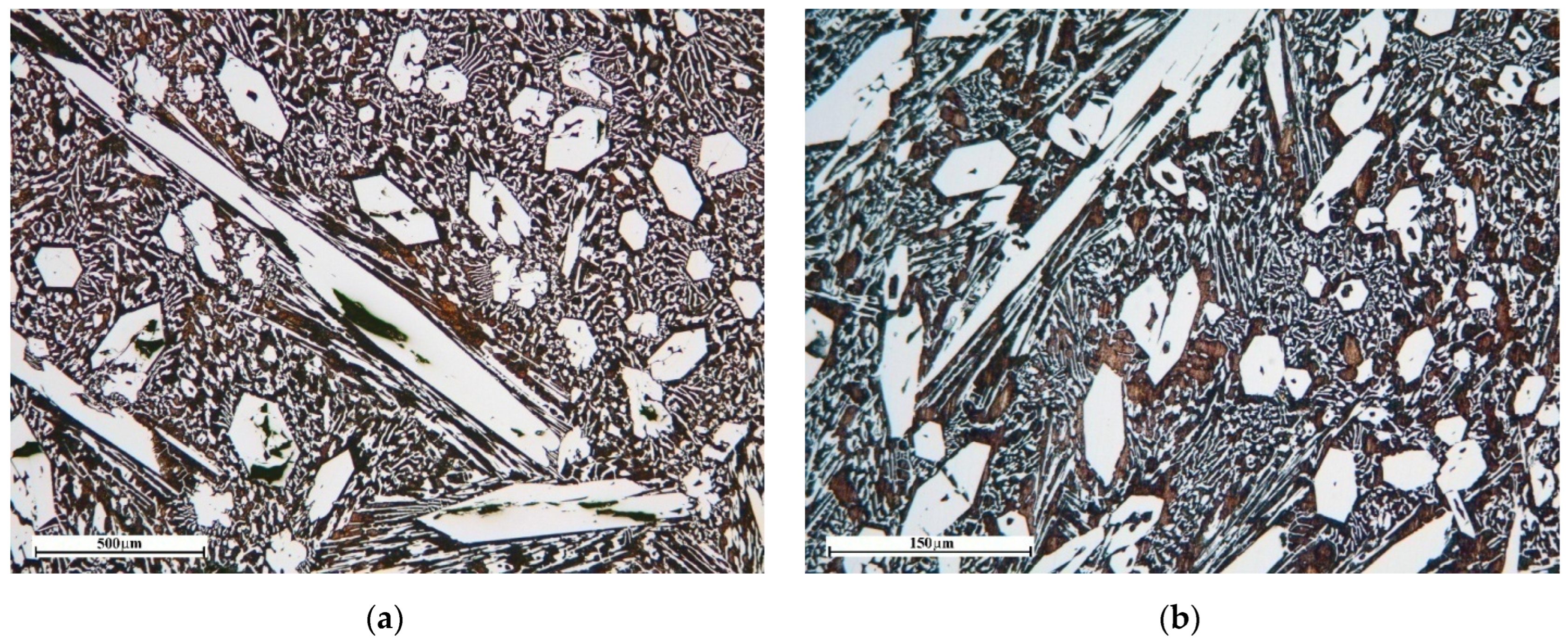
43]. For this purpose, the image was processed by conversion to two-color mode and the separation of primary carbides from eutectic carbides (
Figure 1). The determination of the average cross-sectional area and the average minimum cross-sectional area was required to derive a better estimate of the size of the primary carbides of (Cr, Fe)
7C
3, due to its needle-like structure. When a needle-shaped crystal enters the plane of the section at an angle, the measurement of its length and area cannot be done completely objectively. At the same time, the minimum cross-section characterizes the diameter of the needle-shaped crystal. The structures in the upper, middle and lower parts of the casting were analyzed in the samples of experimental series No.1. In the samples of experimental series No.2, the structure was analyzed at a distance of 2, 6 and 12 mm from the surface of the cylindrical ingot. The results of these measurements were then averaged. The objectivity of the method used for analyzing the structures of castings is evidenced by the measurement results presented in
Figure 2. The dependence of the increase in the proportion of primary carbides on the increase in carbon concentration is close to linear.
The compositions of primary and eutectic carbides and the matrix were determined with the use of a JEOL JXA-8230 microprobe analyzer. The phases of each type were analyzed in at least six points.
Vickers hardness was measured on a HBV-30A hardness tester according to the standard method at a load of 30 kgf using an indenter with a tetrahedral diamond pyramid. The microhardness values of individual phases were measured using a PMT-3 instrument at an indenter load of 50 g.
The microhardness values of the matrix and the primary and eutectic carbides were determined in the first series of experiments, but measurements were taken only for primary carbides in the second series of experiments. This was due to the small sizes of other phases in ingots produced at high cooling rates. A three-point bending test of cast iron ingots was conducted on a Shimadzu AGx 100 kN electromechanical testing machine.
Wear resistance was determined according to the flow chart given in [
31] under GOST 23.208-79, which is a close analog of the ASTM G65 test. Corundum sand was used in the tests; its particle size and shape are shown in
Figure 3. A plate made of 41Cr4 (1.7035) steel in an annealed state with a hardness of 196 HV was taken as a reference sample in the wear tests. The wear was tested at the ambient temperature of 22–25 °C, with the rotation frequency of the rubber roller set as ∅50 mm 1 s
−1, and the number of revolutions as 1800. Accordingly, the wear path was 282.6 m and the test duration was 1800 s; then, the tests were automatically interrupted. After the tests, the wear site was examined at a magnification of up to 5x. If pores and cracks were found in the wear area, the specimen was rejected. Controlled samples were weighed before and after the test on analytical scales with an accuracy to the fourth decimal place. Wear tests were conducted at least 3 times. According to the weighing results, the arithmetic mean value of mass loss in the reference sample and the arithmetic mean value of mass loss in the samples of the material under study were determined. The relative wear resistance of the material under study was calculated according to Formula (1).
where
—arithmetic mean value of the mass loss of the reference samples;
—the arithmetic mean value of mass loss of the tested samples;
ρr, ρt—the densities of the reference and studied materials, g/cm3;
N
r, N
t—the number of revolutions of the roller during the tests of the reference and studied materials.
Figure 3.
Corundum sand used in the tests.
Figure 3.
Corundum sand used in the tests.
A thermal analysis was conducted to determine the effects of cooling rate on the crystallization interval of chromium wear-resistant eutectic cast iron G-X300CrMo27-1 (3.45 wt. %). An STA 449 F3 Jupiter synchronous thermal analyzer was used for this purpose. After the sample was placed in the analyzer chamber, the furnace space was evacuated before heating and purged with inert gas during imaging. Heating was performed at a rate of 0.167 °C/s to 1500 °C, and then the sample was held for 20 min at this temperature. Cooling was conducted at rates of 0.083 (5), 0.167 (10), 0.333 (20), 0.5 (30) and 0.83 (50) °C/s (deg/min).
3. Results
3.1. Metallographic Analysis
The study of the structures of the cast samples showed that they are characteristic of eutectic and hypereutectic HCCI. They are formed of needle-shaped primary carbides (Cr, Fe)7C3 and eutectic γFe + (Cr, Fe)7C3. Needle-shaped (Cr, Fe)7C3 and dendrites γFe occurred in the alloy with a eutectic composition due to the occurrence of non-equilibrium crystallization along with the release of the eutectic substance via the formation of primary carbides. In the studied samples, the eutectic material was characterized by a mixed structure, including colonies of (Cr, Fe)7C3 carbide with needle, skeletal and rosette structures.
The sizes of the phases differed significantly in alloys of identical compositions but obtained at different cooling rates of 0.083–0.117 °C/s and 4.67–5 °C/s, (
Figure 4 and
Table 2). With an increase in cooling rate by a factor of ~50, the average minimum cross-section of primary carbides decreased by a factor of 8.8–11.4, and the decrease in the average cross-section of primary carbides was 47–140-fold. The effect of cooling rate on the size of primary carbides increased with the increase in carbon concentration. The proportion of eutectic carbides did not change significantly with the crystallization rate, and was ~20 vol.% in the alloy with a eutectic composition. The fraction of eutectic carbides was higher in castings with a carbon content of more than 3.0 wt. % derived at cooling rates of 0.083–0.117 °C/s than in castings obtained at 4.67–5 °C/s, by 4–9.4 vol.%. As the carbon concentration in HCCI increases, the fraction of eutectic carbides decreases. This process was also previously observed by Chung et al. [
44]. The cross-sections of eutectic carbides decreased naturally with the increase in cooling rate. Austenite in castings obtained at a cooling rate of 0.083–0.117 °C/s was seen to undergo decomposition in localized areas. The decomposition of austenite increased with increasing carbon content. No traces of austenite decomposition were found in castings obtained at cooling rates of 4.67–5 °C/s.
As can you see in
Figure 5, the shape and size of primary and eutectic carbides did not change after heat treatment. Martensite and granular pearlite were formed in the structure as a result of austenite decomposition in HCCI with carbon concentrations of 2.85, 3.0 and 4.5 wt. %, and martensite was mainly formed in alloys with carbon concentrations of 3.45, 3.75 and 4.2 wt. %, while granular pearlite emerged in local areas.
3.2. Analysis of the Composition of Phases
The analysis of the composition of phases in the HCCI casting containing 4.2 wt. % C showed (
Table 3) that the change in cooling rate in the crystallization interval had practically no effect on the composition of primary carbides of (Cr, Fe)
7C
3. The concentration of Mn did not significantly increase and the Mo concentration decreased with increase in cooling rate. The compositions of eutectic carbides and austenite were significantly more dependent on the cooling rate. Thus, the concentration of Fe increased by ~6 at.%, that of Mn increased by ~0.4 at.%, and Ni increased by 0.3 at.% via dissolving, while the concentration of Cr decreased by ~6.7 at.%, and the contents of C and Mo remained almost unchanged in the composition of eutectic carbides with the increase in cooling rate. Similarly, the contents of Fe, Mn and Mo decreased slightly in the austenite, but Cr content increased by ~3.0 at.%, which may have affected the austenite’s decomposition.
3.3. Mechanical Test Results
Cast HCCI ingots obtained at a cooling rate of 0.083–0.117 °C/s were characterized by the lowest hardness of 540–600 HV (
Figure 6). Among them, cast iron with a carbon content of 3.45 wt. % showed the highest hardness, while the lowest was shown by iron with a carbon content of 3.0 wt. %. Hardness of such castings increased up to 680–810 HV after heat treatment. Castings with 2.85 wt. % C and with 3.75 wt. % C were characterized by the highest hardness, and samples with 4.2 wt. % C and 4.5 wt. % C ß showed the lowest. In general, there is a tendency for the hardness to decrease with the increase in carbon concentration after the heat treatment of the castings.
The hardness values of the casting samples obtained at a cooling rate of 4.67–5 °C/s were in the range 540–650 HV (
Figure 6), and reached a maximum at 3.0 wt. % C (620 HV) and a minimum at 4.2 wt. % C (650 HV). These castings showed a hardness of 640–790 HV after heat treatment. The tendency of hardness to increase with the increase in carbon concentration was observed for these. Accordingly, a casting with a carbon content of 2.85 wt. % was characterized by the lowest hardness, and that with a content of 4.5 wt. % showed the highest.
The microhardness (Hμ) values of the primary carbides in the cast samples obtained at a cooling rate of 0.083–0.117 °C/s decreased uniformly from 1900 to 1780 MPa with increases in the carbon concentration from 2.85 to 4.2 (
Figure 7). The microhardness value was close to 400–425 HV in castings obtained at a cooling rate of 4.67–5 °C/s at a carbon content of 2.85–3.45 wt. %. The hardness of primary carbides gradually increased up to 1790 HV with an increase in carbon content up to 4.5 wt. %.
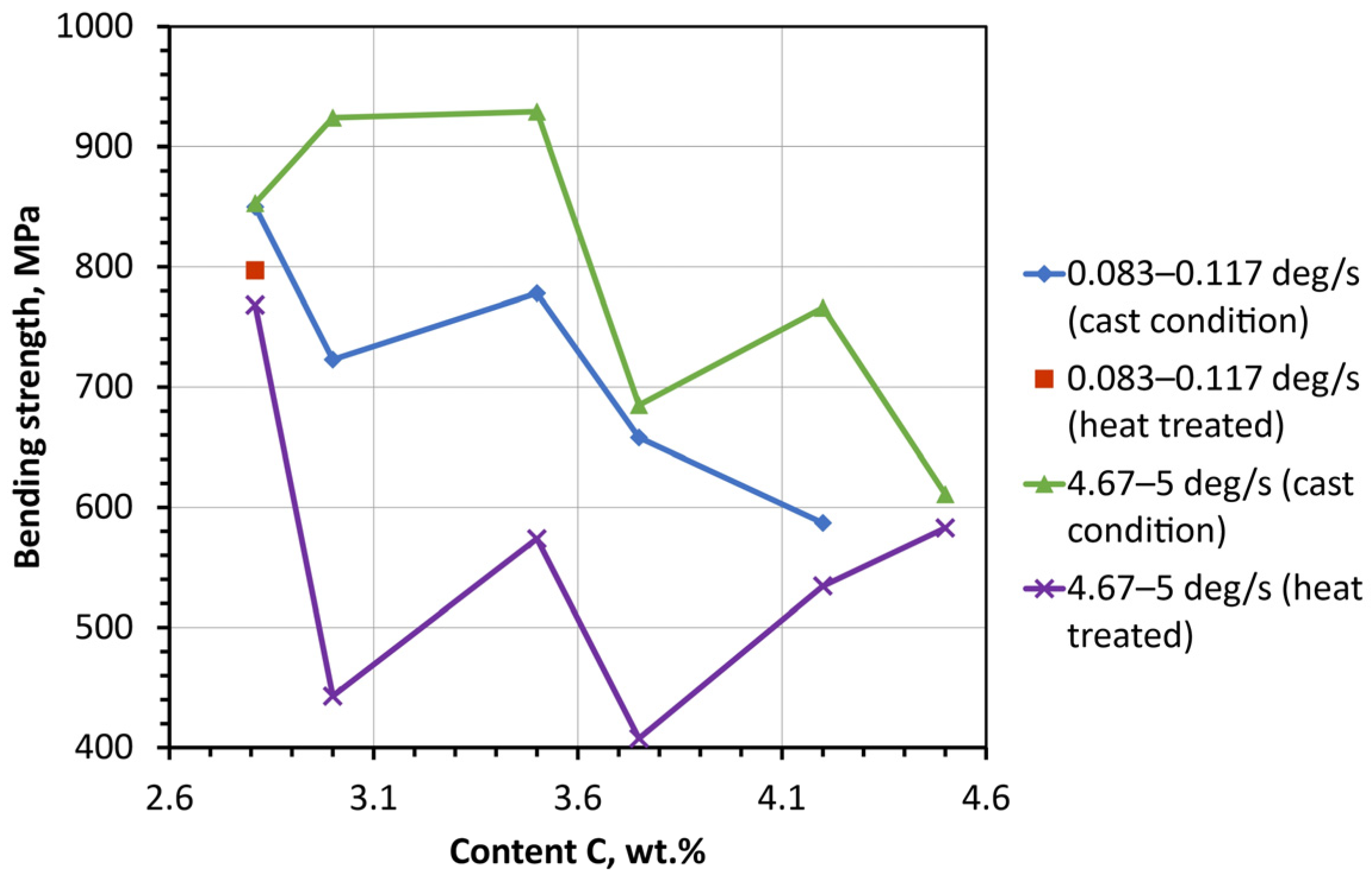
We saw a decrease in bending strength from 820 to 588 Mpa with an increase in carbon concentration in ingots obtained at a cooling rate of 0.083–0.117 °C/s (
Figure 8). The cast ingots obtained at the cooling rate of 4.67–5 °C/s were characterized by a higher ultimate strength of 600–930 MPa. The highest strength was exhibited by castings with carbon contents of 3.0 and 3.45 wt. %, and the lowest by those with 4.5 wt.% contents.
Ingots obtained at cooling rates of 4.67–5 °C/s underwent a significant decrease in strength after heat treatment. Thus, the drop in strength amounted to 400, 350 and 290 MPa at carbon concentrations of 3.0, 3.45 and 3.75 wt. %. Ingots with carbon concentrations of 3.0 and 3.75 wt. % showed the lowest tensile strength. There was a gradual increase in strength with greater carbon contents.
The relative wear resistance values of HCCI castings obtained at cooling rates of 0.083–0.117 °C/s, with carbon concentrations ranging from 3.45 to 4.2 wt. %, were at their minimum (of 2.5–6.16) in the cast state (
Figure 9). The highest wear resistance was shown by castings with a content of 3.0 wt. %, with a value of 10.5. The wear resistance values of castings with a eutectic composition increased significantly (by ~100% to 17.6), as did castings with a eutectic composition and contents of 3.45, 3.75 and 4.2 wt. %C (by 135, 510 and 133%, respectively), after the heat treatment of ingots. The alloy with a carbon concentration of 3.75% displayed a wear resistance of 15.2.
Cast specimens obtained at a crystallization rate of 4.67–5 °C/s and increased carbon concentration exhibited nonhomogeneous changes in wear resistance (
Figure 9). The maximum wear resistance was shown by castings with 3.45 wt. % C, with a value of 28.4, and other than this, the wear resistance was not significantly higher compared to castings obtained at cooling rates of 0.083–0.117 °C/s. The minimum wear resistance was shown by the casting with 3.75 wt. % C (4.28), as was also seen under slow cooling. The wear resistance values of castings with 2.8, 3.0, 3.45, 4.2 and 4.5 wt. % C decreased significantly (by 63%, 21%, 51%, 69% and 94%, respectively) after heat treatment. And the wear resistance of the casting with 3.75 wt. % C content increased significantly (by 363% to 19.8).
3.4. Thermal Analysis
The effect of the cooling rate of HCCI cast iron (3.45 wt. % C) on the crystallization onset temperature and eutectic point can be traced by observing the shifts in the peaks on the DTA curves shown in
Figure 10.
The crystallization onset temperature shifted no more than 21 °C, and the eutectic point shifted ~30 °C towards lower temperatures, which adheres to the results of the thermal analysis performed with a cooling rate that increased from 0.083 to 0.83 °C/s (from 5 to 50 °C/min).
4. Discussion
Chromium white cast iron can be considered a metal matrix composite material, since it is formed from a metal plastic matrix and a high-modulus filler with an endogenous formation. The plasticity of such a discrete filler is close to 0%.
Analysis of the results of studying the structure and measuring the elemental composition of primary and eutectic carbides showed that in the range of cooling rates from 0.083–0.117 deg/s to 4.67–5 deg/s at a head temperature of 2.85 to 4.5 wt.% at crystallization in As a result of cast iron G-X300CrMo27-1, only carbide (Cr, Fe)7C3 and austenite were used.
The studies show that the changes in carbon concentration and cooling rate in the crystallization interval had a significant effect on the properties of castings derived from HCCI hypereutectic cast irons both when it was in the cast state and after heat treatment. However, the changes seen in these properties cannot be totally explained by the change in the structure and elemental composition of phases, because these are poorly correlated.
Changes in castings occurring with increases in the cooling rate (from 0.083–0.117 °C/s to 4.67–5 °C/s) were associated with a significant decrease in the sizes of primary and eutectic carbides, and changes in the elemental composition of eutectic carbides and austenite. Thus, the average cross-sectional areas of primary carbides with such increased cooling rates decreased by a factor of 47–140. Consequently, the number of reinforcing particles per unit area increased by such a factor. A similar change occurred when using eutectic carbides, and this naturally resulted in a decrease in the distance between reinforcing particles. The observed decrease in Cr concentration in eutectic carbides by ~6 at.%, and the insignificant increase in Mn and Ni concentrations, certainly may have reduced their hardness, but the increase in Cr concentration by ~3 at.% in austenite obviously increased the strength of the matrix. Unfortunately, it was not possible to measure the microhardness in castings obtained at cooling rates of 4.67–5 °C/s due to the small sizes of these phases. These changes in structure and composition should have clear and significantly effects in terms of improving the perturbation-sensitive properties, such as hardness, strength and abrasion resistance. However, a comparison of the characteristics of castings with identical compositions but obtained at different cooling rates indicates that the actual difference in properties is not so significant.
The non-significant change in the composition of primary carbides and the noticeable change in the composition of eutectic carbides can be explained by the difference in their crystallization temperature ranges. Thus, ref. [
45] indicated that the formation of carbides takes place via diffusion; therefore, the chemical composition depends on the influence of thermokinetic and thermodynamic factors (deviation from equilibrium). The shift of the eutectic point to the region of lower temperatures with an increase in the cooling rate in the crystallization range naturally leads to a shift towards iron in the phase diagram. This explains the increase in the concentration of iron in the composition of eutectic carbide in the casting obtained with a cooling rate of 4.67–5 °C/s. At the same time, the insignificant change in the concentration of primary carbides with increasing cooling rates is explained by their formation within a wide crystallization temperature range, which, according to the thermal data, expands with an increasing cooling rate. The decrease in the concentration of Mo in primary carbides and the increases in the concentrations of Mo, Mn, and Ni in secondary carbides an increasing cooling rate can be explained both by the difference in the diffusion coefficients of these elements, and by the difference in the formation mechanisms of these carbides.
An increase in the carbon content of the alloy leads to an increase in the content of (Cr, Fe)7C3 carbide, which is significantly harder than austenite. This should have increased the hardness of the castings, but no such increase actually occurred in their crystallization at cooling rates of 0.083–0.117 °C/s, and the hardness increased only with carbon contents of 4.2 and 4.5 wt. % C with cooling rates of 4.67–5 °C/s.
The tensile strength values of cylindrical ingots with a hypereutectic composition decreased with increases in carbon content. Despite the increase in the proportion of hardening phase, this decrease can be explained by the fact that negative stresses replace positive ones in the three-point bending test along the cross-section of the sample. It is known that brittle materials subjected to a force that creates negative stresses fracture at significantly lower loads than in the case of positive stresses.
The increase in carbide content in the composition of HCCI castings, together with the increase in the microhardness of primary carbides (in the case of cooling at a rate of 4.67–5 °C/s) with the increase in carbon content, should increase their resistance to abrasive wear. However, this has actually only been observed in a narrow concentration range for cast irons with a hypereutectic composition of up to 3.0 wt. % C and from 4.2 to 4.5 wt. % C. In this case, the increase in wear resistance was incommensurably less than the changes in the structures and properties of phases in the castings. The study of the wear surface structure showed that, in the case of castings obtained at a cooling rate of 0.083–0.117 °C/s, a mixed mechanism of carbide phase destruction took place. Some of the large primary carbides are destroyed with the formation of chips and cracks, while the remaining ones, as well as the majority of eutectic carbides, gradually wear together with the austenitic matrix. The destruction of primary carbides due to their cracking is significantly lower during the wearing of samples cut from ingots obtained at a cooling rate of 4.67–5 °C/s; however, the resistance to abrasive wear with the increase in solid phase content did not increase significantly, and showed minimal values at a carbon content of 3.75 wt. % C.
The observed inconsistencies in the changes in the structure, the compositions of phases and the mechanical properties of castings with increases in carbon concentration may be explained by the increase in the sizes of primary carbides and the decrease in the proportion of eutectic carbides. However, these discrepancies are obviously related to the appearance of internal stresses in primary and (probably) eutectic carbides (Cr, Fe)
7C
3. This can explain the significant difference in microhardness between primary carbides (
Figure 7) obtained in castings at different cooling rates, as well as the change in the microhardness of carbides following the increase in carbon content in the castings.
The changes that occur in the castings after heat treatment are predominantly related to the decomposition of austenite. Changes in properties may also occur due to the development of interfacial diffusion interactions. The process of austenite decomposition naturally results in an increase in the hardness of the matrix and a decrease in its plasticity. This is confirmed by the results obtained. However, changes in the properties of castings due to heat treatment are caused by both the carbon content and the cooling rate. Thus, the decrease in the strength of castings (4.67–5 °C/s) along with the increase in hardness can be explained by the formation of a stronger but more brittle matrix. The formation of martensite in conjunction with an increase in the proportion of hard but brittle primary carbides results in a sharp decrease in the strength of castings containing 3, 3.45 and 3.75 wt. % C. At the same time, the decomposition of austenite with the formation of granular pearlite resulted in an insignificant decrease in strength, as well as a noticeable increase in hardness.
The shapes of the curves of the dependence of relative wear resistance on the carbon content in castings after heat treatment are similar for castings obtained at cooling rates of 0.083–0.117 °C/s and 4.67–5 °C/s. The abrasion resistance of the slowly crystallized castings was higher, while it was lower before heat treatment. This indicates that the structure and phase composition of the matrix plays a role in determining the resistance to the abrasive surface failure of HCCI castings. Small carbide sizes and small carbide spacings benefit wear resistance only in the case of a soft matrix, but as the matrix hardens, the effect of a reduction in the carbide size in the castings decreases. The analysis of the wear surface’s structure has indicated that a sharp decrease in the wear resistance of ingots obtained at a cooling rate of 0.083–0.117 °C/s occurs when their carbon content reaches 4.2 wt. % C. This is caused by the cracking of primary carbides and their gradual destruction due to the spalling of large fragments (
Figure 11). In the case of tests performed on ingots obtained at cooling rates of 4.67–5 °C/s, wear occurs without spalling, but the small sizes of the carbides accelerates matrix fracturing and their subsequent shearing.
It is obvious that other factors, such as the presence of non-metallic inclusions, have effects besides those considered, such as how the structure affects the mechanical properties of castings of high-chromium cast iron. However, as studies [
46] have shown, their prevalence is not particularly great, and accordingly, their contribution to the reduction in mechanical properties is not significant. In this regard, it is necessary to develop technological approaches that ensure a reduction in internal stresses in primary and eutectic carbides (Cr, Fe)
7C
3 and increases in the strength and toughness of the matrix, in order to improve the characteristics of HHCCI castings. This can be achieved both by alloying and by the application of certain heat treatment regimes.
5. Conclusions
The average sizes and the proportions of primary carbides (from 8–12 to 28–35 vol.%) increased, and the proportion of eutectic carbides decreased, with increase in carbon concentration (from 2.85 to 4.5 wt. %) in the composition of high-chromium cast iron of G-X300CrMo27-1 grade. The decrease in the fraction of eutectic carbides with the increase in carbon concentration in wear-resistant, high-chromium cast iron castings obtained at a cooling rate of 4.67–5 °C/s was more significant compared to castings obtained at a cooling rate of 0.083–0.117 °C/s. This change in structure had a significant effect on the hardness, strength and wear resistance of the castings.
The effect of the cooling rate of HCCI melt during the crystallization interval on mechanical properties is related to both the change in the size of phases (primary and eutectic carbides) and the change in their composition. The compositions of eutectic carbides and austenite changed most significantly with the increase in cooling rate (from 0.083–0.117 °C/s to 4.67–5 °C/s).
The changes in the structure of HCCI castings with eutectic compositions showing increased carbon contents and following increases in cooling rate during the crystallization interval do not fully agree with the changes seen in the properties of such castings when in the cast state. It is assumed that internal stresses developing in primary and eutectic carbides have a significant influence on the final properties of castings.
The significant increases in the hardness and abrasive wear resistance of castings after heat treatment indicate a large contribution of the matrix to the development of HCCI’s properties. The formation of the martensitic matrix provides the greatest wear resistance to cast irons, but the lowest strength, and the formation of a granular pearlite structure increases hardness, but wear resistance decreases accordingly.