Composite of Cellulose-Nanofiber-Reinforced Cellulose Acetate Butyrate: Improvement of Mechanical Strength by Cross-Linking of Hydroxyl Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparatio
2.3. Samples Characterization
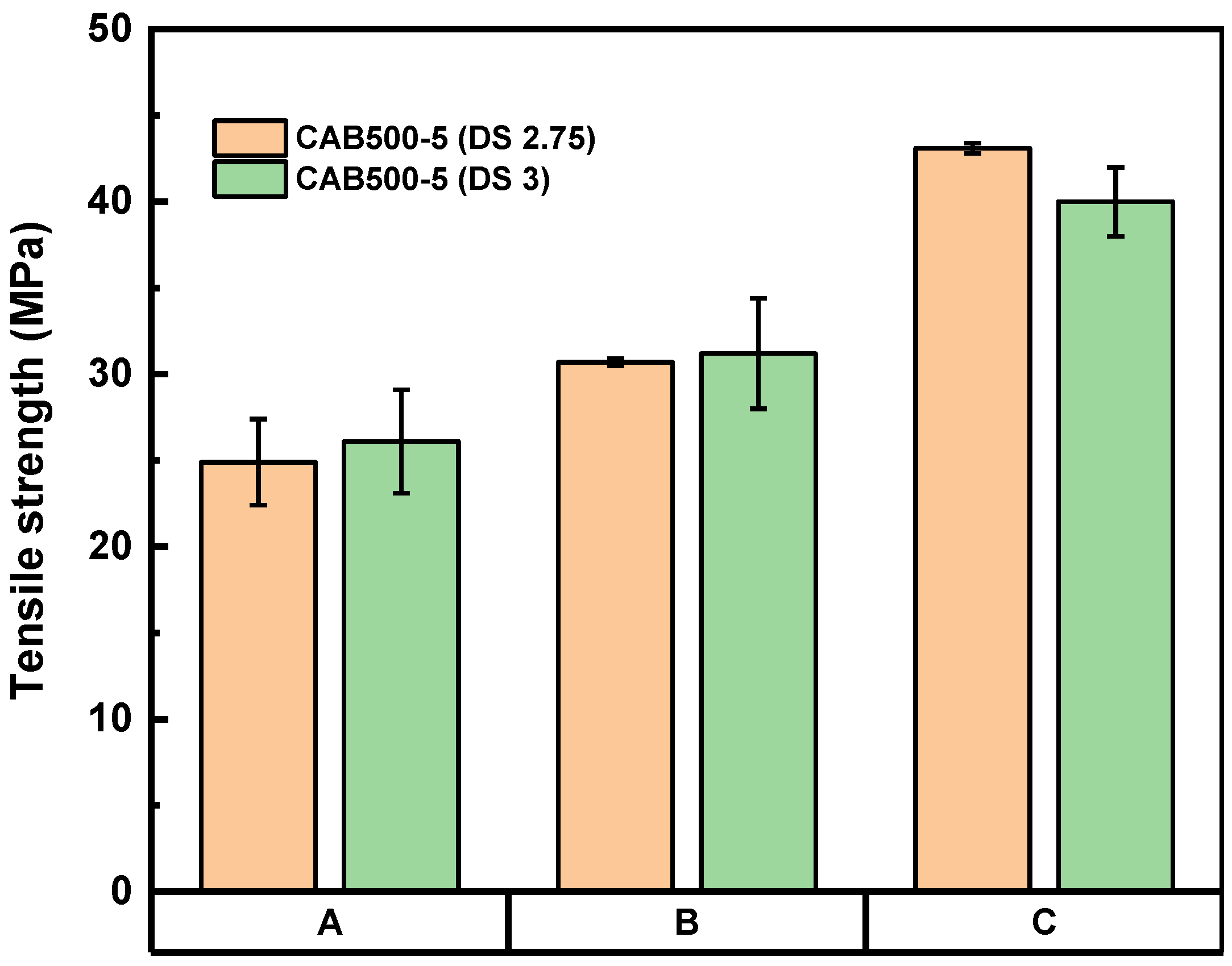
3. Results and Discussion
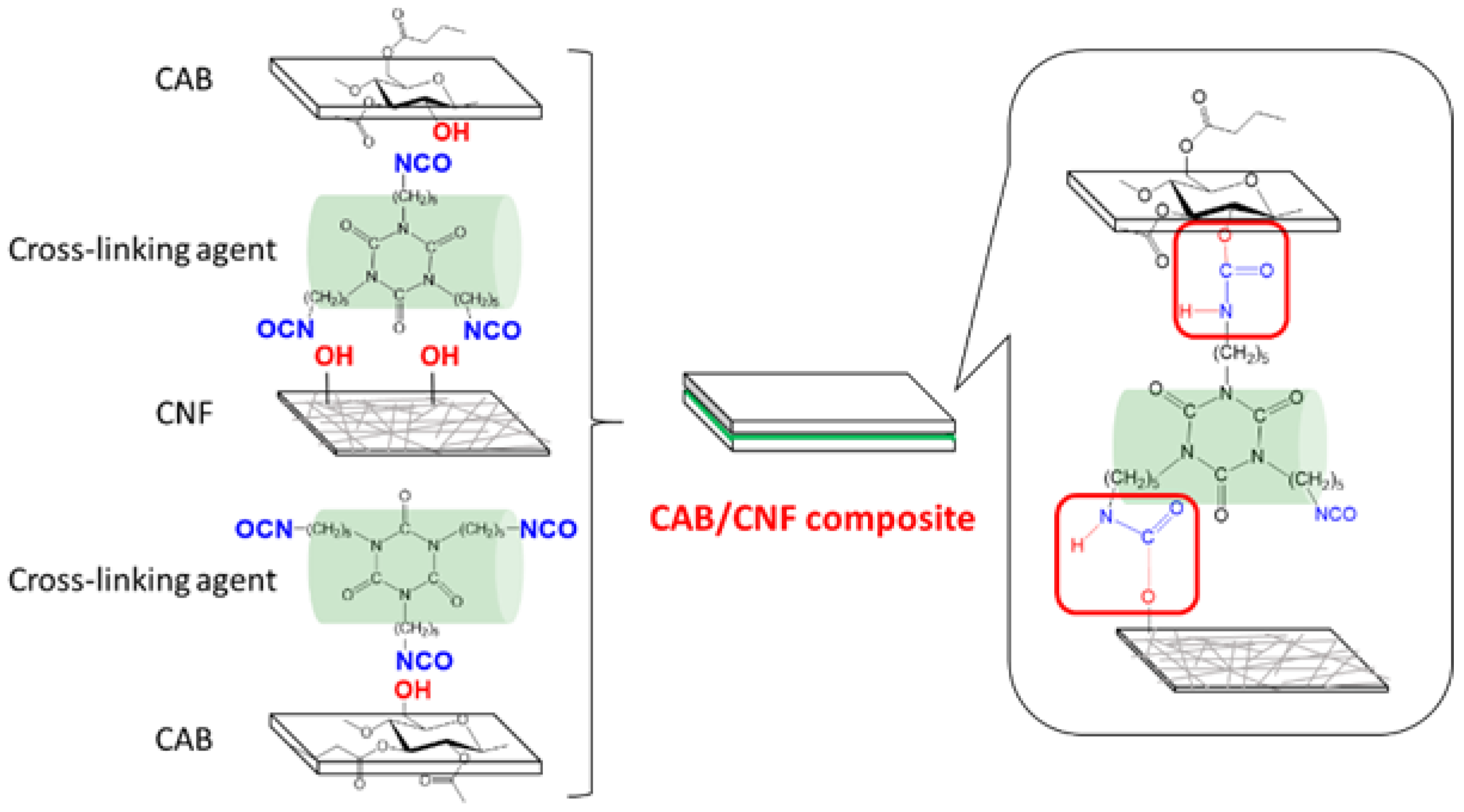
3.1. CAB/CNF Laminate Preparation
3.2. CAB/A-CNF/D376N Laminate Preparation
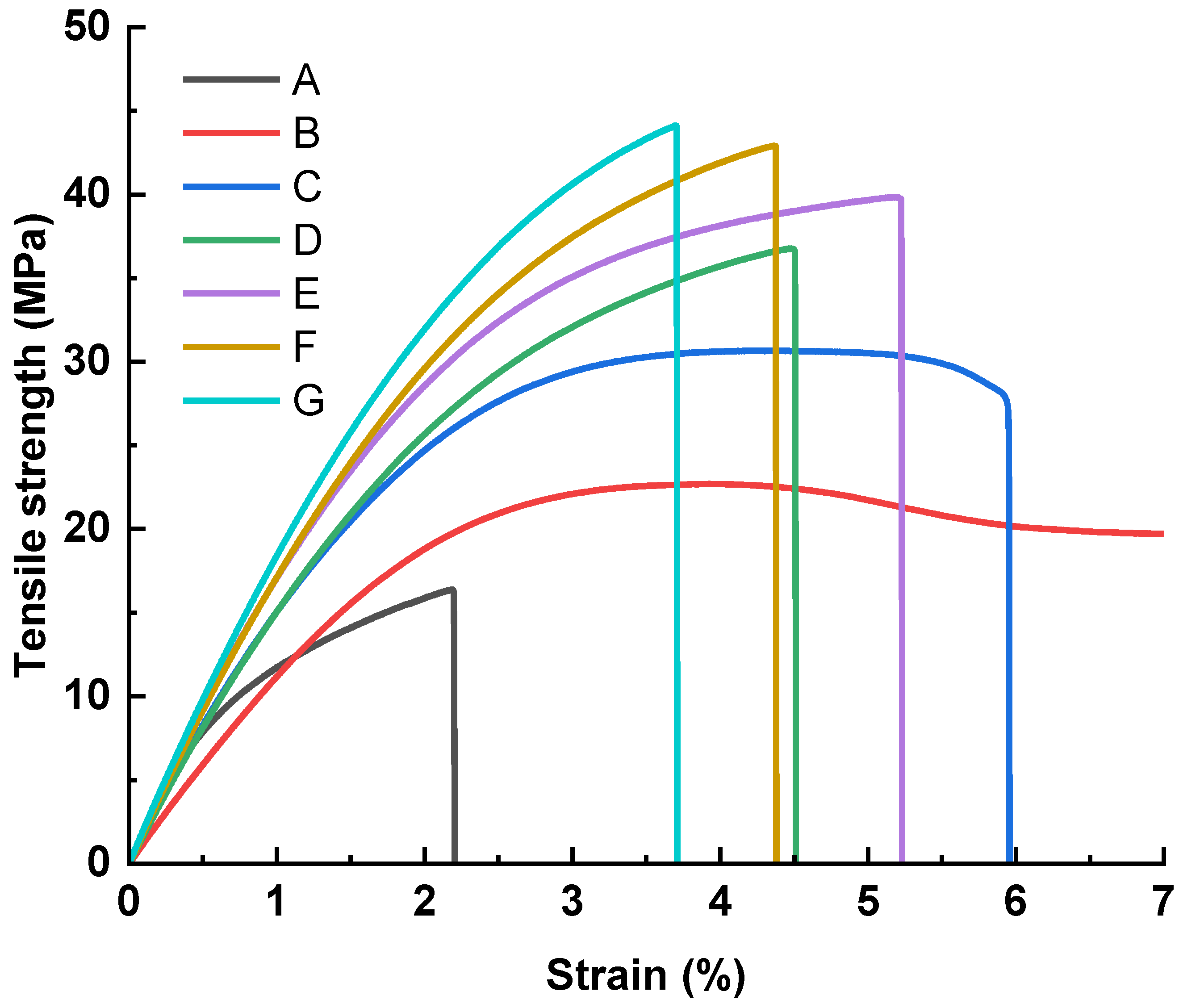
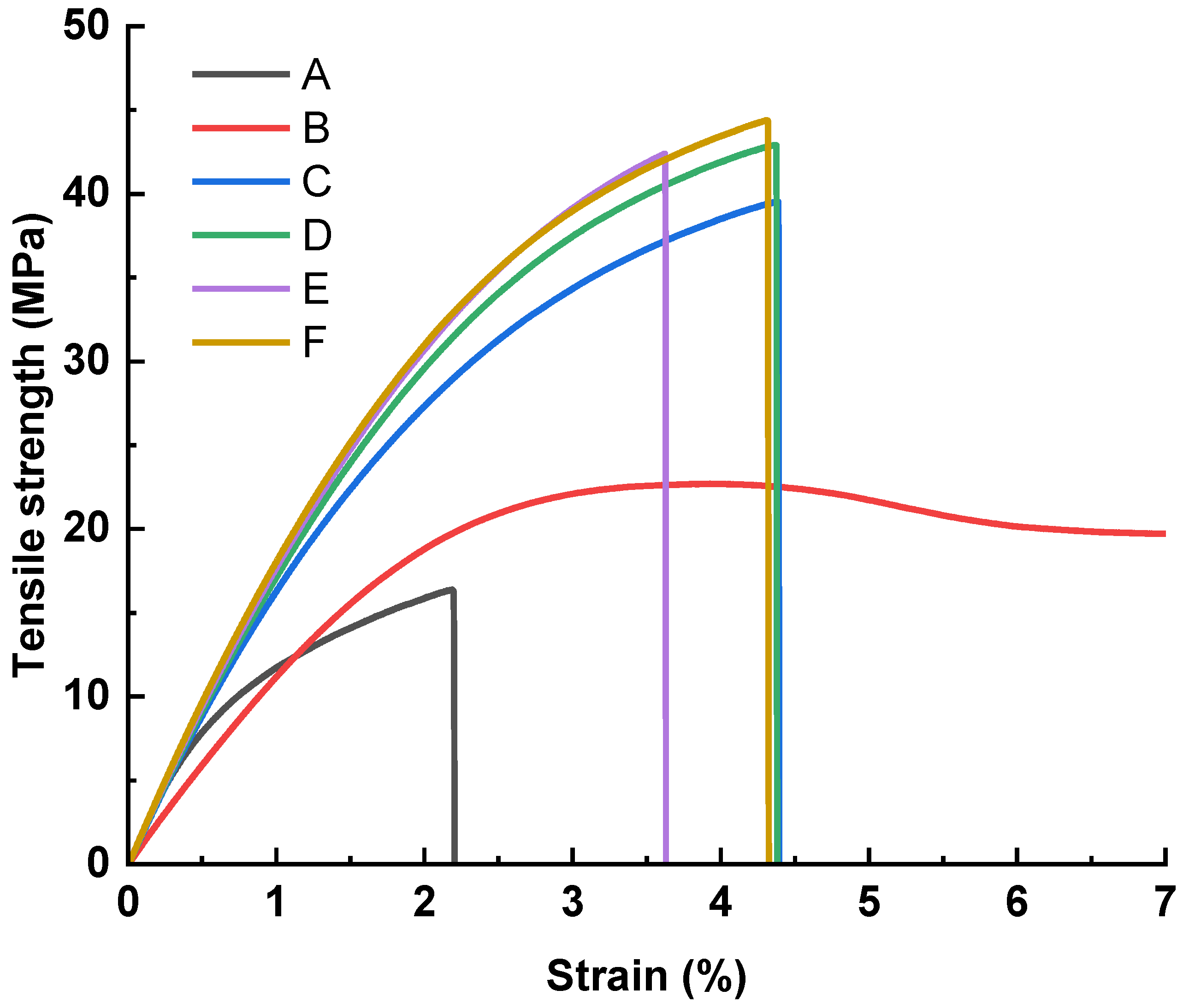
3.3. Influence of Hot-Press Time
3.4. Influence of Hot-Press Pressure
3.5. Flexural Test Results
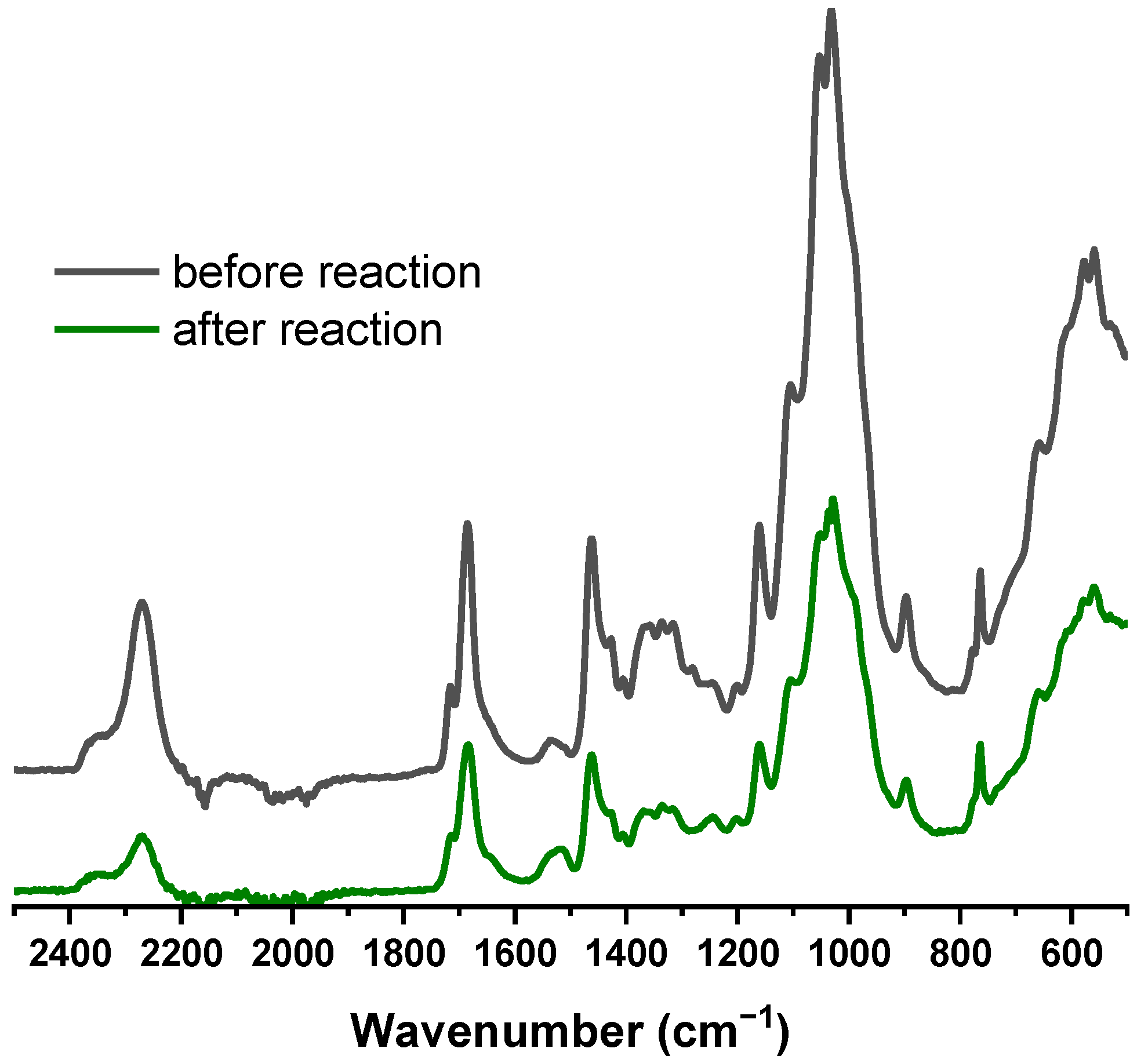
3.6. Investigation of Cross-Linking between CNFs
3.7. Confirmation of Cross-Linking Reaction at the CAB/A-CNF Interface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andrew, J.J.; Dhakal, H.N. Sustainable biobased composites for advanced applications: Recent trends and future opportunities—A critical review. Compos. Part C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Jonoobi, M.; Mathew, A.P.; Abdi, M.M. A Comparison of Modified and Unmodified Cellulose Nanofiber Reinforced Polylactic Acid (PLA) Prepared by Twin Screw Extrusion. J. Polym. Environ. 2012, 20, 991–997. [Google Scholar] [CrossRef]
- Wu, J.; Dong, Z.; Li, X.; Li, P.; Wei, J.; Hu, M.; Geng, L.; Peng, X. Constructing acid-resistant chitosan/cellulose nanofibrils composite membrane for the adsorption of methylene blue. J. Environ. Chem. Eng. 2022, 10, 107754. [Google Scholar] [CrossRef]
- Drzal, L.T.; Madhukar, M. Fibre-matrix adhesion and its relationship to composite mechanical properties. J. Mater. Sci. 1993, 28, 569–610. [Google Scholar] [CrossRef]
- Dufresne, A. Processing of Polymer Nanocomposites Reinforced with Polysaccharide Nanocrystals. Molecules 2010, 15, 4111–4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Her, K.; Jeon, S.H.; Lee, S.; Shim, B.S. Esterification of Cellulose Nanofibers with Valeric Acid and Hexanoic Acid. Macromol. Res. 2020, 28, 1055–1063. [Google Scholar] [CrossRef]
- Beaumont, M.; Winklehner, S.; Veigel, S.; Mundigler, N.; Gindl-altmutter, W.; Potthast, A.; Rosenau, T. Simple process to surface-acetylated cellulose. Green Chem. 2020, 22, 5605–5609. [Google Scholar] [CrossRef]
- Navarro, J.R.G.; Edlund, U. Surface-Initiated Controlled Radical Polymerization Approach to Enhance Nanocomposite Integration of Cellulose Nanofibrils. Biomacromolecules 2017, 18, 1947–1955. [Google Scholar] [CrossRef]
- Roy, D.; Guthrie, J.T.; Perrier, S. Graft Polymerization: Grafting Poly(styrene) from Cellulose via Reversible Addition−Fragmentation Chain Transfer (RAFT) Polymerization. Macromolecules 2005, 38, 10363–10372. [Google Scholar] [CrossRef]
- Goussé, C.; Chanzy, H.; Cerrada, M.L.; Fleury, E. Surface silylation of cellulose microfibrils: Preparation and rheological properties. Polymer 2004, 45, 1569–1575. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Duarte, A.P.; Ben Salah, A.; Gandini, A. Modification of cellulosic fibres with functionalised silanes: Development of surface properties. Int. J. Adhes. Adhes. 2004, 24, 43–54. [Google Scholar] [CrossRef]
- Laitinen, O.; Suopajärvi, T.; Österberg, M.; Liimatainen, H. Hydrophobic, Superabsorbing Aerogels from Choline Chloride-Based Deep Eutectic Solvent Pretreated and Silylated Cellulose Nanofibrils for Selective Oil Removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, F.; Berglund, L.A. Toward Semistructural Cellulose Nanocomposites: The Need for Scalable Processing and Interface Tailoring. Biomacromolecules 2018, 19, 2341–2350. [Google Scholar] [CrossRef]
- Szabó, L.; Milotskyi, R.; Fujie, T.; Tsukegi, T.; Wada, N.; Ninomiya, K.; Takahashi, K. Short Carbon Fiber Reinforced Polymers: Utilizing Lignin to Engineer Potentially Sustainable Resource-Based Biocomposites. Front. Chem. 2019, 7, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.E.; Shim, S.B.; Park, J.H.; Chung, I. Interfacial Properties and Melt Processability of Cellulose Acetate Propionate Composites by Melt Blending of Biofillers. Polymers 2022, 14, 4286. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, D.; Syre, P.; Niska, K.O. All cellulose nanocomposites produced by extrusion. J. Biobased Mater. Bioenergy 2007, 1, 367–371. [Google Scholar] [CrossRef]
- Wada, N.; Fujie, T.; Sasaki, R.; Matsushima, T.; Takahashi, K. Direct synthesis of a robust cellulosic composite from cellulose acetate and a nanofibrillated bacterial cellulose sol. Polym. J. 2022, 54, 735–740. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Jonoobi, M.; Aitomäki, Y.; Mathew, A.P.; Oksman, K. Thermoplastic polymer impregnation of cellulose nanofibre networks: Morphology, mechanical and optical properties. Compos. Part A Appl. Sci. Manuf. 2014, 58, 30–35. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winandy, J.E.; Krzysik, A.M. Thermal degradation of wood fibers during hot-pressing of MDF composites. Part I, Relative effects and benefits of thermal exposure. Wood Fiber Sci. 2007, 39, 450–461. [Google Scholar]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar] [CrossRef]



| /kg/m3 | Porosity/% | |
|---|---|---|
| Untreated CNF sheet | 878.7 | 41.4 |
| Acetone-treated CNF sheet | 677.8 | 58.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milotskyi, R.; Serizawa, R.; Yanagisawa, K.; Sharma, G.; Ito, E.R.D.; Fujie, T.; Wada, N.; Takahashi, K. Composite of Cellulose-Nanofiber-Reinforced Cellulose Acetate Butyrate: Improvement of Mechanical Strength by Cross-Linking of Hydroxyl Groups. J. Compos. Sci. 2023, 7, 130. https://doi.org/10.3390/jcs7030130
Milotskyi R, Serizawa R, Yanagisawa K, Sharma G, Ito ERD, Fujie T, Wada N, Takahashi K. Composite of Cellulose-Nanofiber-Reinforced Cellulose Acetate Butyrate: Improvement of Mechanical Strength by Cross-Linking of Hydroxyl Groups. Journal of Composites Science. 2023; 7(3):130. https://doi.org/10.3390/jcs7030130
Chicago/Turabian StyleMilotskyi, Romain, Ryo Serizawa, Kaoru Yanagisawa, Gyanendra Sharma, Elisabeth Rada Desideria Ito, Tetsuo Fujie, Naoki Wada, and Kenji Takahashi. 2023. "Composite of Cellulose-Nanofiber-Reinforced Cellulose Acetate Butyrate: Improvement of Mechanical Strength by Cross-Linking of Hydroxyl Groups" Journal of Composites Science 7, no. 3: 130. https://doi.org/10.3390/jcs7030130
APA StyleMilotskyi, R., Serizawa, R., Yanagisawa, K., Sharma, G., Ito, E. R. D., Fujie, T., Wada, N., & Takahashi, K. (2023). Composite of Cellulose-Nanofiber-Reinforced Cellulose Acetate Butyrate: Improvement of Mechanical Strength by Cross-Linking of Hydroxyl Groups. Journal of Composites Science, 7(3), 130. https://doi.org/10.3390/jcs7030130







