Porous NiMoO4-NrGO as a Battery-Like Electrode Material for Aqueous Hybrid Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
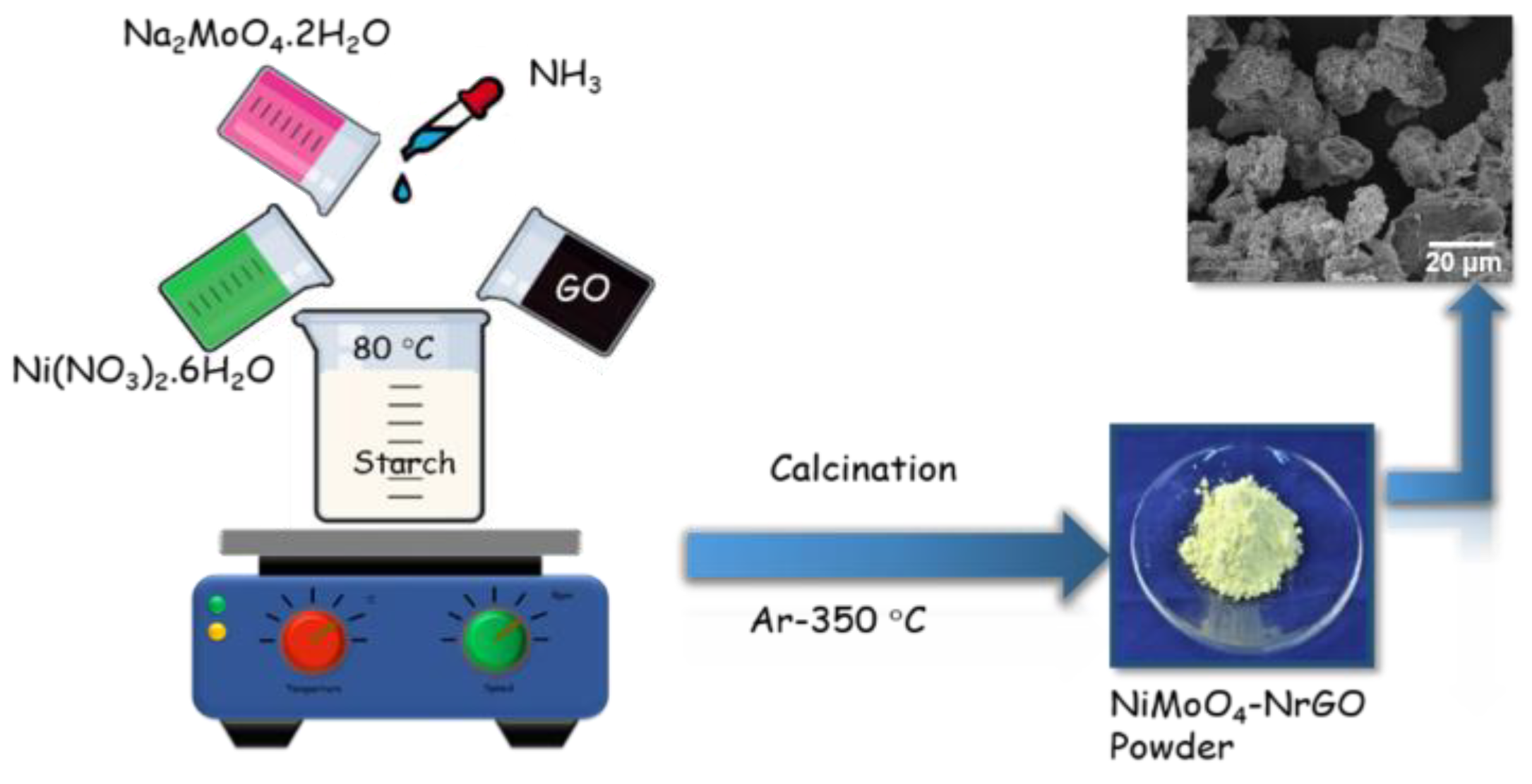
2.2. Preparation of NiMoO4-rGO and NiMoO4/N-Doped rGO Hybrid Nanocomposite
2.3. Preparation of NiMoO4-rGO and NiMoO4-NrGO Hybrid Electrodes Using Ni Foam Current Collectors
2.4. Material Characterization
2.5. Electrochemical Investigations
3. Results and Discussion
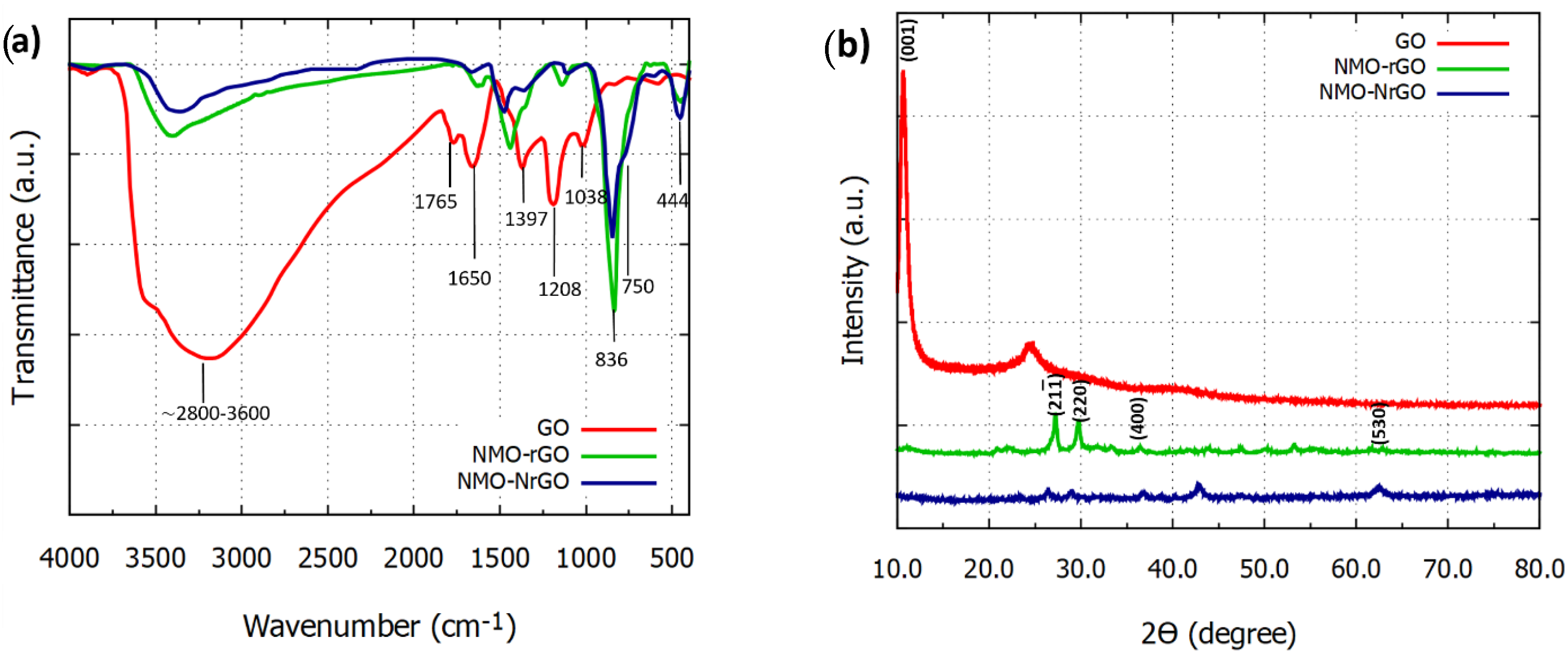
3.1. Material Characterization of the Electrodes
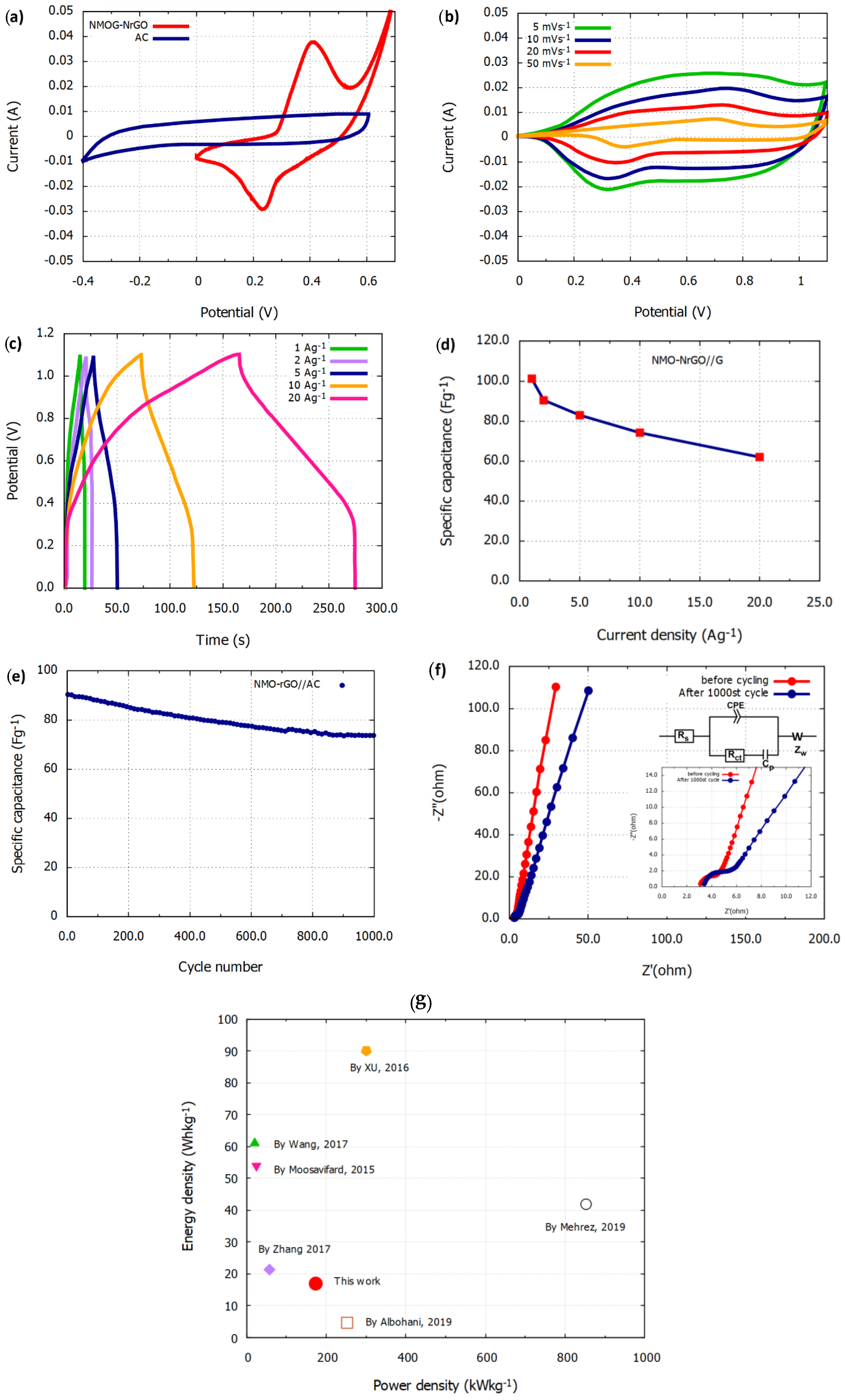
3.2. Electrochemical Properties of NMO-rGO and NMO-NrGO Electrodes
3.3. Electrochemical Properties of an NMO-NrGO//AC Asymmetric Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Li, T.; Lu, M.; Chen, Y.; Cui, Y.; Gao, J.; Qian, G. Tuning the electronic structure of a metal–organic framework for an efficient oxygen evolution reaction by introducing minor atomically dispersed ruthenium. Carbon Energy 2023, 5, e265. [Google Scholar] [CrossRef]
- Shown, I.; Ganguly, A.; Chen, L.-C.; Chen, K.H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Mahalingam, P. Syntheses and applications of carbon nanotubes and their composites. Carbon App. 2013, 22, 495–537. [Google Scholar]
- Wang, K.; Chen, C.; Li, Y.; Hong, Y.; Wu, H.; Zhang, C.; Zhang, Q. Insight into Electrochemical Performance of Nitrogen-Doped Carbon/NiCo-Alloy Active Nanocomposites. Small 2023, 2300054. [Google Scholar] [CrossRef]
- Yue, T.; Shen, B.; Gao, P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: A review. Renew. Sustain. Energy Rev. 2022, 158, 112131. [Google Scholar] [CrossRef]
- Veeresh, S.; Ganesha, H.; Nagaraju, Y.S.; Vijeth, H.; Vandana, M.; Basappa, M.; Devendrappa, H. Graphene ox-ide/cobalt oxide nanocomposite for high-performance electrode for supercapacitor application. J. Energy Storage 2022, 52, 104715. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, R.S.; Atchudan, R.; Kim, H.-J.; Yi, M.; Samyn, L.M.; de Barros, A.L.F. Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC). Catalysts 2022, 12, 375. [Google Scholar] [CrossRef]
- Karimi, F.; Korkmaz, S.; Karaman, C.; Karaman, O.; Kariper, A. Engineering of GO/MWCNT/RuO2 ternary aerogel for high-performance supercapacitor. Fuel 2022, 329, 125398. [Google Scholar] [CrossRef]
- Jinlong, L.; Meng, Y.; Suzuki, K.; Miura, H. Synthesis of CoMoO4@RGO nanocomposites as high-performance supercapacitor electrodes. Microporous Mesoporous Mater. 2017, 242, 264–270. [Google Scholar] [CrossRef]
- Prabhu, S.; Maruthapandi, M.; Durairaj, A.; Arun Kumar, S.; Luong, J.H.; Ramesh, R.; Gedanken, A. Performances of Co2+-substituted NiMoO4 nanorods in a solid-state hybrid supercapacitor. ACS Appl. Energy Mater. 2023, 6, 1321–1331. [Google Scholar] [CrossRef]
- Jayasubramaniyan, S.; Balasundari, S.; Rayjada, P.A.; Satyanarayana, N.; Muralidharan, P. Microwave hydrothermal synthesis of α-MnMoO4 nanorods for high electrochemical performance supercapacitors. RSC Adv. 2018, 8, 22559–22568. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Yang, B.; Song, W.; Liu, Q.; Jia, F.; Qin, S.; Chen, W.; Zhang, Z.; Li, J. Three-dimensional graphene skeletons supported nickel molybdate nanowire composite as novel ultralight electrode for supercapacitors. Mater. Lett. 2016, 164, 401–404. [Google Scholar] [CrossRef]
- Wu, F.; Liao, Q.; Cao, F.; Li, L.; Zhang, Y. Non-noble bimetallic NiMoO4 nanosheets integrated Si photoanodes for highly efficient and stable solar water splitting. Nano Energy 2017, 34, 8–14. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, J.; Chen, W.; Zhao, Y.; Mu, X.; Zhang, Z.; Pan, X.; Xie, E. Constructing highly-efficient electron transport channels in the 3D electrode materials for high-rate supercapacitors: The case of NiCo2O4@NiMoO4 hierarchical nanostructures. Chem. Eng. J. 2017, 307, 687–695. [Google Scholar] [CrossRef]
- Liu, T.; Chai, H.; Jia, D.; Su, Y.; Wang, T.; Zhou, W. Rapid microwave-assisted synthesis of mesoporous NiMoO4 nanorod/reduced graphene oxide composites for high-performance supercapacitors. Electrochim. Acta 2015, 180, 998–1006. [Google Scholar] [CrossRef]
- Kianpour, G.; Salavati-Niasari, M.; Emadi, H. Sonochemical synthesis and characterization of NiMoO4 nanorods. Ultrason. Sonochem. 2013, 20, 418–424. [Google Scholar] [CrossRef]
- Umapathy, V.; Neeraja, P.; Manikandan, A.; Ramu, P. Synthesis of NiMoO4 nanoparticles by sol–gel method and their structural, morphological, optical, magnetic and photocatlytic properties. Trans. Nonferr. Met. Soc. China 2017, 27, 1785–1793. [Google Scholar] [CrossRef]
- Sharma, G.P.; Pala, R.G.S.; Sivakumar, S. Ultrasmall NiMoO4 robust nanoclusters-active carbon composite for high performance extrinsic pseudocapacitor. Electrochim. Acta 2019, 318, 607–616. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, H.; Wang, Q.; Wang, Q.; Zhou, G. Nitrogen-doped carbon/NiMoO4 nanospheres assembled by nanosheets and ultrasmall nanoparticles for supercapacitors. Chem. Phys. Lett. 2019, 728, 215–223. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Q.; Zhang, X. A Novel Hierarchical Flower-like NiMoO4 for Supercapacitors. Chem. Lett. 2018, 47, 1213–1215. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Chen, Y.; Rui, K.; Mao, H.; Dou, S.X.; Sun, W. Iron-Doped Nickel Molybdate with Enhanced Oxygen Evolution Kinetics. Chem. A Eur. J. 2019, 25, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, Y.; Zhang, Q. Metal–Organic Frameworks Constructed from Iron-Series Elements for Supercapacitors. Small Struct. 2022, 3, 2100115. [Google Scholar] [CrossRef]
- Qian, H.; Jinlong, L.; Tongxiang, L.; Chen, W. In Situ Synthesis of Mesoporous NiMoO4 on Ball Milled Graphene for High Performance Supercapacitors. J. Electrochem. Soc. 2017, 164, E173–E179. [Google Scholar] [CrossRef]
- Prakash, N.G.; Dhananjaya, M.; Narayana, A.L.; Hussain, O.M. High performance one dimensional α-MoO3 nanorods for supercapacitor applications. Ceram. Int. 2018, 44, 9967–9975. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, S. NiO-CNT composite for high performance supercapacitor electrode and oxygen evolution reaction. Electrochim. Acta 2018, 283, 327–337. [Google Scholar] [CrossRef]
- Park, G.D.; Hong, J.H.; Lee, J.-K.; Kang, Y.C. Yolk–shell-structured microspheres composed of N-doped-carbon-coated NiMoO4 hollow nanospheres as superior performance anode materials for lithium-ion batteries. Nanoscale 2019, 11, 631–638. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K. Converting Corncob to Activated Porous Carbon for Supercapacitor Application. Nanomaterials 2018, 8, 181. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Ab Rahim, M.H.; Jose, R. Synthesis and characterization of MnCo2O4 cuboidal microcrystals as a high performance psuedocapacitor electrode. Alloy. Compd. 2016, 656, 707–713. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- Srivastava, M.; Uddin, E.; Singh, J.; Kim, N.H.; Lee, J.H. Preparation and characterization of self-assembled layer by layer NiCo2O4–reduced graphene oxide nanocomposite with improved electrocatalytic properties. J. Alloys Compd. 2014, 590, 266–276. [Google Scholar] [CrossRef]
- Fayer, M.D. Ultrafast Infrared Vibrational Spectroscopy; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef]
- Li, Y.; Jian, J.; Fan, Y.; Wang, H.; Yu, L.; Cheng, G.; Sun, M. Facile one-pot synthesis of a NiMoO4/reduced graphene oxide composite as a pseudocapacitor with superior performance. RSC Adv. 2016, 6, 69627–69633. [Google Scholar] [CrossRef]
- de Moura, A.P.; de Oliveira, L.H.; Rosa, I.L.; Xavier, C.S.; Lisboa-Filho, P.N.; Li, M.S.; Varela, J.A. Structural, optical, and magnetic properties of NiMoO4 nanorods prepared by microwave sintering. Sci. World J. 2015. [Google Scholar] [CrossRef]
- Arshadi Rastabi, S.; Sarraf Mamoory, R.; Blomquist, N.; Phadatare, M.; Olin, H. Synthesis of a NiMoO4/3D-rGO nanocomposite via starch medium precipitation method for supercapacitor performance. Batteries 2020, 6, 5. [Google Scholar] [CrossRef]
- Yousefi, R.; Mahmoudian, M.; Sa΄aedi, A.; Cheraghizade, M.; Jamali-Sheini, F.; Azarang, M. Effect of annealing temperature and graphene concentrations on photovoltaic and NIR-detector applications of PbS/rGO nanocomposites. Ceram. Int. 2016, 42, 15209–15216. [Google Scholar] [CrossRef]
- Arshadi Rastabi, S.; Sarraf Mamoory, R.; Dabir, F.; Blomquist, N.; Phadatare, M.; Olin, H. Synthesis of Ni-MoO4/3D-rGO nanocomposite in alkaline environments for supercapacitor electrodes. Crystals 2019, 9, 31. [Google Scholar] [CrossRef]
- Bankar, P.K.; Ratha, S.; More, M.A.; Late, D.J.; Rout, C.S. Enhanced field emission performance of NiMoO4 nanosheets by tuning the phase. Appl. Surf. Sci. 2017, 418, 270–274. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Khan, A. Aerosol-Assisted Chemical Vapor Deposition Growth of NiMoO4 Nanoflowers on Nickel Foam as Effective Electrocatalysts toward Water Oxidation. ACS Omega 2021, 6, 31339–31347. [Google Scholar] [CrossRef]
- Li, G.L.; Qiao, X.Y.; Miao, Y.Y.; Wang, T.Y.; Deng, F. Synergistic Effect of N-NiMoO4/Ni Heterogeneous Interface with Oxygen Vacancies in N-NiMoO4/Ni/CNTs for Superior Overall Water Splitting. Small 2023, 2207196. [Google Scholar] [CrossRef]
- Azarang, M.; Shuhaimi, A.; Yousefi, R.; Sookhakian, M. Effects of graphene oxide concentration on optical properties of ZnO/RGO nanocomposites and their application to photocurrent generation. J. Appl. Phys. 2014, 116, 084307. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Al Wadaani, F. Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles. Molecules 2018, 23, 273. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, W.; Niu, H.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J. Ultrahigh energy density battery-type asymmetric su-percapacitors: NiMoO4 nanorod-decorated graphene and graphene/Fe2O3 quantum dots. Nano Res. 2018, 11, 4744–4758. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; Shamsi, J.; Ayazpour, M. 2D high-ordered nanoporous NiMoO4 for high-performance super-capacitors. Ceram. Int. 2015, 41, 1831–1837. [Google Scholar] [CrossRef]
- Arshadi Rastabi, S.; Sarraf-Mamoory, R.; Razaz, G.; Blomquist, N.; Hummelgård, M.; Olin, H. Treatment of Ni-MoO4/nanographite nanocomposite electrodes using flexible graphite substrate for aqueous hybrid supercapacitors. PLoS ONE 2021, 16, e0254023. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, P.; Zhang, H.; Yu, X.; Zhu, J.; Li, Q.; Wang, T. NiMoO4 nanowires supported on Ni foam as novel advanced electrodes for supercapacitors. J. Mater. Chem. A 2013, 1, 9024–9027. [Google Scholar] [CrossRef]
- Vlad, A.; Singh, N.; Rolland, J.; Melinte, S.; Ajayan, P.M.; Gohy, J.-F. Hybrid supercapacitor-battery materials for fast electrochemical charge storage. Sci. Rep. 2014, 4, 4315. [Google Scholar] [CrossRef]
- Nakanishi, H.; Kikuta, I.; Kawabata, Y.; Norisuye, T.; Tran-Cong-Miyata, Q.; Segawa, H. Fast Ion and Electron Transport in a Supercapacitor Based on Monolithic Nanowire-Array Electrodes Prepared from a Defect-Free Anodic Alu-minium Oxide Mold. Adv. Mater. Interfaces 2015, 2, 1500354. [Google Scholar] [CrossRef]
- Guo, D.; Luo, Y.; Yu, X.; Li, Q.; Wang, T. High performance NiMoO4 nanowires supported on carbon cloth as advanced electrodes for symmetric supercapacitors. Nano Energy 2014, 8, 174–182. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Gilliam, R.; Graydon, J. A review of specific conductivities of potassium hydroxide solutions for various concentrations and temperatures. Int. J. Hydrogen Energy 2007, 32, 359–364. [Google Scholar] [CrossRef]
- Budhiraju, V.S.; Kumar, R.; Sharma, A.; Sivakumar, S. Structurally stable hollow mesoporous graphitized carbon nanofibers embedded with NiMoO4 nanoparticles for high performance asymmetric supercapacitors. Electrochim. Acta 2017, 238, 337–348. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, D.; Pei, S.; Ye, L.; Geng, S.; Lian, J. Rational fabrication of nanosheet-dewy NiMoO4/Ni3S2 nanohybrid for efficient hybrid supercapacitor. J. Alloys Compd. 2019, 783, 399–408. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Liu, J.; Li, C.; Mao, F.-F.; Wu, H.; Zhang, Q. Enhancing the Performance of a Battery–Supercapacitor Hybrid Energy Device Through Narrowing the Capacitance Difference Between Two Electrodes via the Utilization of 2D MOF-Nanosheet-Derived Ni@Nitrogen-Doped-Carbon Core–Shell Rings as Both Negative and Positive Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 47482–47489. [Google Scholar] [CrossRef]
- Ramya, A.V.; Thomas, R.; Balachandran, M. Mesoporous onion-like carbon nanostructures from natural oil for high-performance supercapacitor and electrochemical sensing applications: Insights into the post-synthesis sonochemical treatment on the electrochemical performance. Ultrason. Sonochem. 2021, 79, 105767. [Google Scholar] [CrossRef]
- Yue, X.; Chen, Z.; Xiao, C.; Song, G.; Zhang, S.; He, H. Synthesis of CNT@ CoS/NiCo Layered Double Hydroxides with Hollow Nanocages to Enhance Supercapacitors Performance. Nanomaterials 2022, 12, 3509. [Google Scholar] [CrossRef]
- Yazar; Sibel; Arvas, M.B.; Sahin, Y. An ultrahigh-energy density and wide potential window aqueous electrolyte supercapacitor built by polypyrrole/aniline 2-sulfonic acid modified carbon felt electrode. Int. J. Energy Res. 2022, 46, 8042–8060. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zheng, Y.; Zhang, Y.; Hu, X.; Xu, T. NiCo2S4@NiMoO4 Core-Shell Heterostructure Nanotube Arrays Grown on Ni Foam as a Binder-Free Electrode Displayed High Electrochemical Performance with High Capacity. Nanoscale Res. Lett. 2017, 12, 412. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, G.; Du, K.; Zhao, X.; Liu, M.; Wang, S.; Zhou, Y.; An, C.; Zhang, J. Ni Foam-Supported Carbon-Sheathed NiMoO4 Nanowires as Integrated Electrode for High-Performance Hybrid Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 5964–5971. [Google Scholar] [CrossRef]
- Albohani, S.; Sundaram, M.M. Egg shell membrane template stabilises formation of β-NiMoO4 nanowires and en-hances hybrid supercapacitor behaviour. Mater. Lett. 2019, 236, 64–68. [Google Scholar] [CrossRef]
- Mehrez JA, A.; Owusu, K.A.; Chen, Q.; Li, L.; Hamwi, K.; Luo, W.; Mai, L. Hierarchical MnCo2O4@NiMoO4 as Free-Standing Core-Shell Nanowire Arrays with Synergistic Effect for Enhanced Supercapacitor Performance. Inorg. Chem. Front. 2019, 1, 857–865. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Q.; Wei, T.; Zhao, Y.; Zhang, X. Enhanced energy storage activity of NiMoO4 modified by graphitic carbon nitride. J. Mater. Sci. Mater. Electron. 2019, 30, 5109–5119. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshadi-Rastabi, S.; Sarraf-Mamoory, R.; Razaz, G.; Blomquist, N.; Örtegren, J.; Olin, H. Porous NiMoO4-NrGO as a Battery-Like Electrode Material for Aqueous Hybrid Supercapacitors. J. Compos. Sci. 2023, 7, 217. https://doi.org/10.3390/jcs7060217
Arshadi-Rastabi S, Sarraf-Mamoory R, Razaz G, Blomquist N, Örtegren J, Olin H. Porous NiMoO4-NrGO as a Battery-Like Electrode Material for Aqueous Hybrid Supercapacitors. Journal of Composites Science. 2023; 7(6):217. https://doi.org/10.3390/jcs7060217
Chicago/Turabian StyleArshadi-Rastabi, Shahrzad, Rasoul Sarraf-Mamoory, Ghadir Razaz, Nicklas Blomquist, Jonas Örtegren, and Håkan Olin. 2023. "Porous NiMoO4-NrGO as a Battery-Like Electrode Material for Aqueous Hybrid Supercapacitors" Journal of Composites Science 7, no. 6: 217. https://doi.org/10.3390/jcs7060217
APA StyleArshadi-Rastabi, S., Sarraf-Mamoory, R., Razaz, G., Blomquist, N., Örtegren, J., & Olin, H. (2023). Porous NiMoO4-NrGO as a Battery-Like Electrode Material for Aqueous Hybrid Supercapacitors. Journal of Composites Science, 7(6), 217. https://doi.org/10.3390/jcs7060217





