Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Ethylene Succinate)/Hemp Fiber Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(ethylene succinate) (PESu) and Its Composites with Hemp Fibers
2.3. Characterization Methods
2.3.1. Thermogravimetric Analysis (TGA)
2.3.2. Pyrolysis–Gas Chromatography/Mass Spectrometry (Py–GC/MS)
3. Results
3.1. Thermogravimetric Analysis
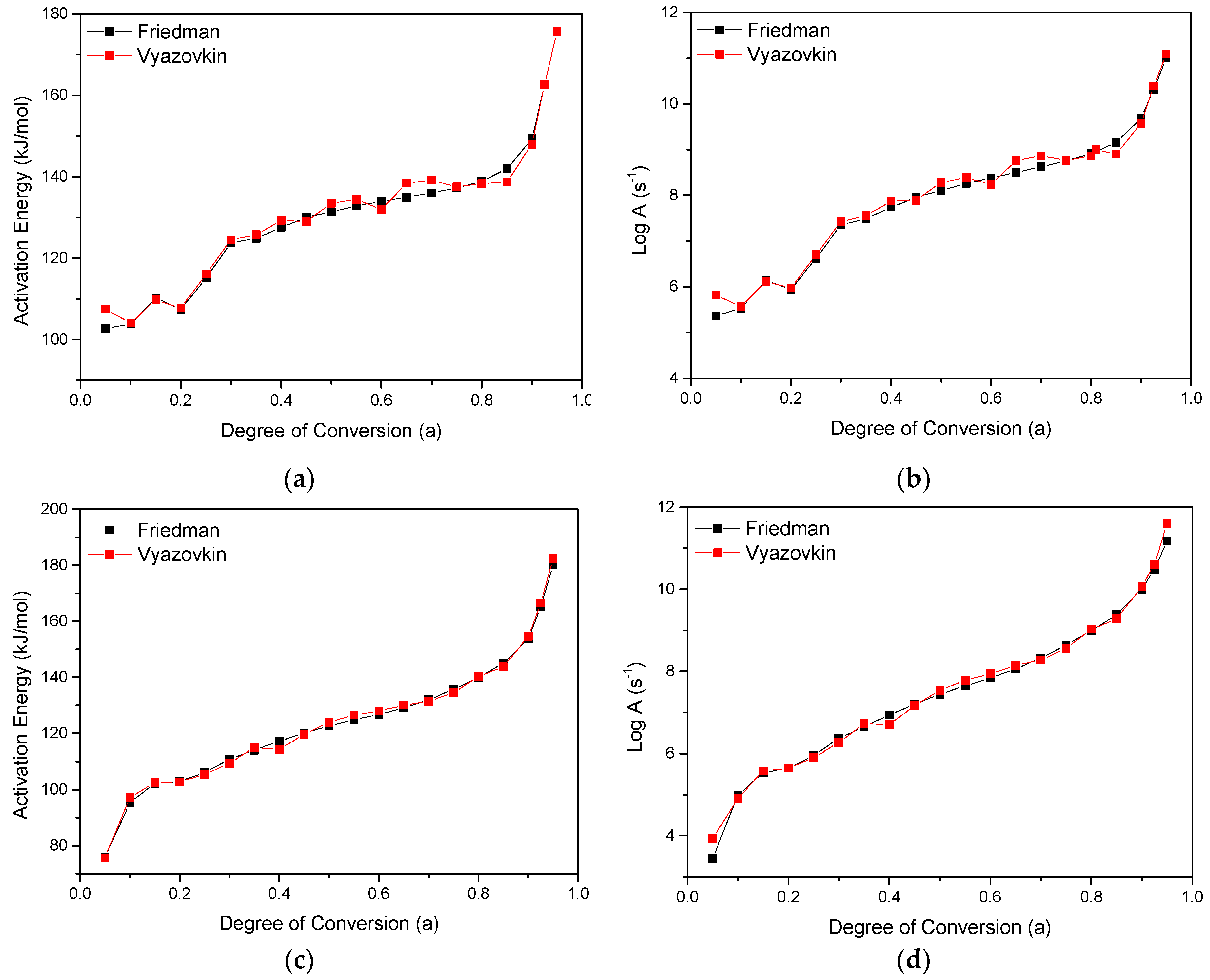
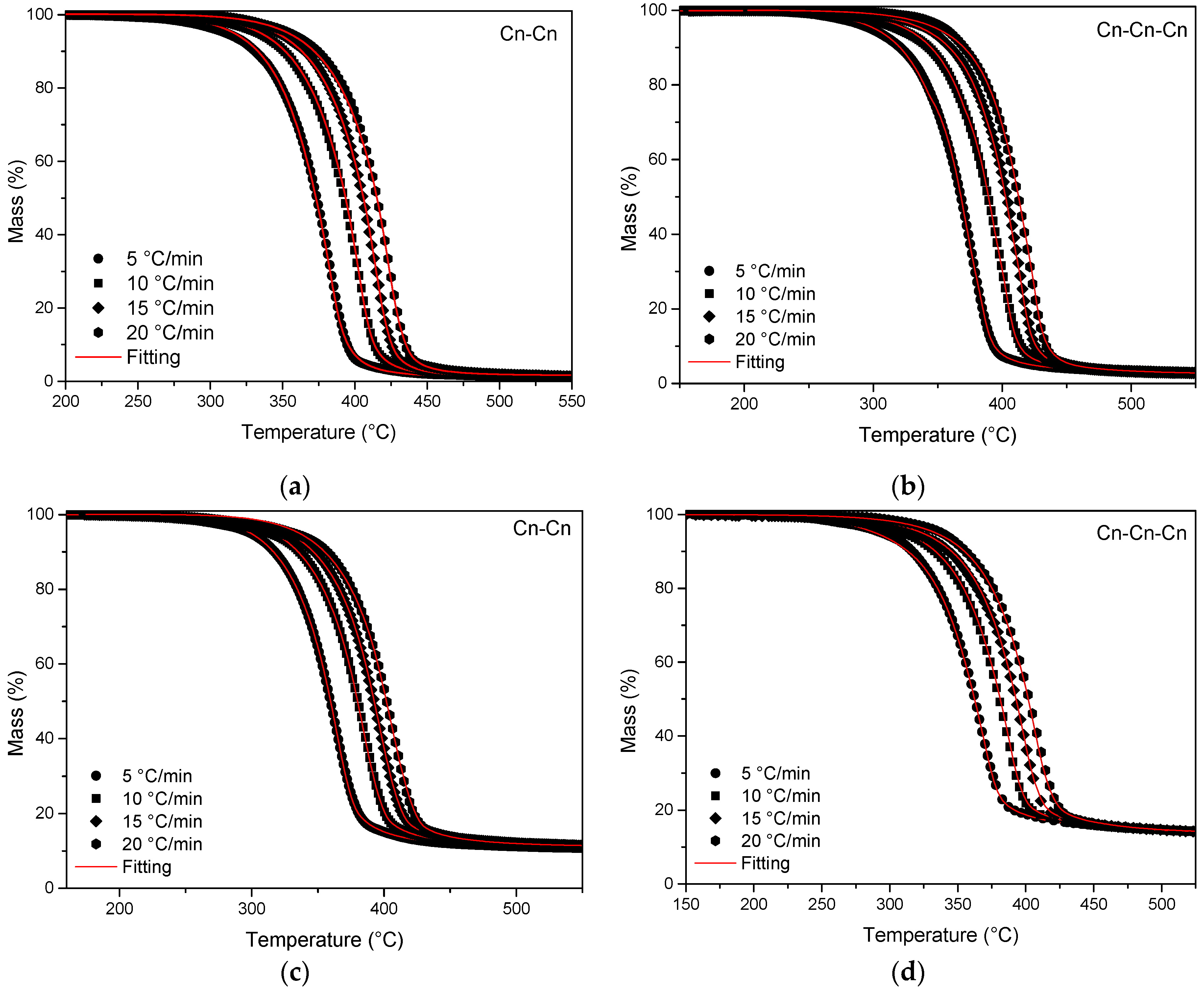
3.2. Kinetic Analysis Based on Thermogravimetric Data—Isoconversional Methods
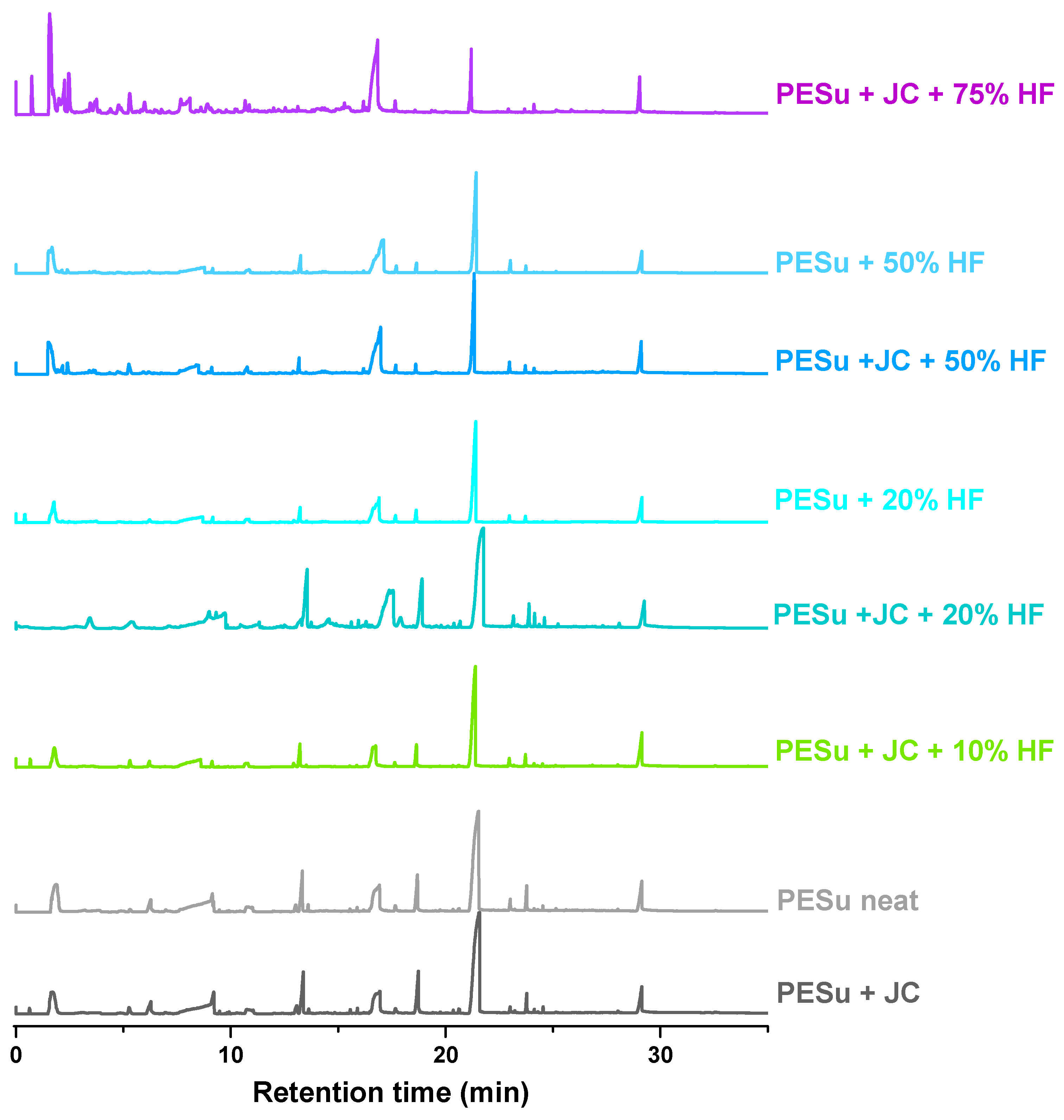
3.3. Pyrolysis–Gas Chromatography/Mass Spectrometry (Py–GC/MS) Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoelscher, F.; Cardoso, P.B.; Candiotto, G.; Guindani, C.; Feuser, P.; Araújo, P.H.H.; Sayer, C. In Vitro Degradation and Cytotoxicity Response of Biobased Nanoparticles Prepared by Thiol-Ene Polymerization in Miniemulsion. J. Polym. Environ. 2021, 29, 3668–3678. [Google Scholar] [CrossRef]
- He, Z.; Feng, Y.; Wang, C.; Yang, J.; Tan, T.; Yang, J. Structure and Properties of New Biodegradable Elastomers Composed of Poly(Ethylene Succinate)-Based Poly(Ether Ester)s and Poly(Lactic Acid). J. Appl. Polym. Sci. 2023, 140, 1–15. [Google Scholar] [CrossRef]
- Keridou, I.; Franco, L.; del Valle, L.J.; Martínez, J.C.; Funk, L.; Turon, P.; Puiggalí, J. Hydrolytic and Enzymatic Degradation of Biobased Poly(4-Hydroxybutyrate) Films. Selective Etching of Spherulites. Polym. Degrad. Stab. 2021, 183, 109451. [Google Scholar] [CrossRef]
- Rowe, M.D.; Eyiler, E.; Walters, K.B. Hydrolytic Degradation of Bio-Based Polyesters: Effect of PH and Time. Polym. Test. 2016, 52, 192–199. [Google Scholar] [CrossRef]
- Lv, X.; Luo, F.; Zheng, L.; Niu, R.; Liu, Y.; Xie, Q.; Song, D.Q.; Zhang, Y.C.; Zhou, T.; Zhu, S. Biodegradable Poly(Butylene Succinate-Co-Butylene Furandicarboxylate): Effect of Butylene Furandicarboxylate Unit on Thermal, Mechanical, and Ultraviolet Shielding Properties, and Biodegradability. J. Appl. Polym. Sci. 2022, 139, e53122. [Google Scholar] [CrossRef]
- Burelo, M.; Gutiérrez, S.; Treviño-Quintanilla, C.D.; Cruz-Morales, J.A.; Martínez, A.; López-Morales, S. Synthesis of Biobased Hydroxyl-Terminated Oligomers by Metathesis Degradation of Industrial Rubbers SBS and PB: Tailor-Made Unsaturated Diols and Polyols. Polymers 2022, 14, 4973. [Google Scholar] [CrossRef]
- Chen, S.; Zou, R.; Li, L.; Shang, J.; Lin, S.; Lan, J. Preparation of Biobased Poly(Propylene 2,5-Furandicarboxylate) Fibers: Mechanical, Thermal and Hydrolytic Degradation Properties. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric Sustainable Materials and Their Emerging Applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Papadimitriou, S.A.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization and Enzymatic Degradation of Novel Poly (e -Caprolactone-Co-Propylene Succinate) Copolymers. Eur. Polym. J. 2008, 44, 2356–2366. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Roumeli, E.; Chrissafis, K.; Lioutas, C.; Triantafyllidis, K.; Bikiaris, D.; Boccaccini, A.R. Thermal Degradation Kinetics and Decomposition Mechanism of PBSu Nanocomposites with Silica-Nanotubes and Strontium Hydroxyapatite Nanorods. Phys. Chem. Chem. Phys. 2014, 16, 4830–4842. [Google Scholar] [CrossRef]
- Lv, X.; Haitao, L.; Zhengxiang, W.; Ruixue, N.; Yi, L.; Wei, Y.; Zheng, L. Synthesis of Biodegradable Polyester–Polyether with Enhanced Hydrophilicity, Thermal Stability, Toughness, and Degradation Rate. Polymers 2022, 14, 4895. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, R.D.; Ainali, N.M.; Christodoulou, E.; Nikolaidis, N.; Lambropoulou, D.A.; Papageorgiou, G.Z. Thermal Stability and Decomposition Mechanism of Poly(Alkylene Succinate)S. Macromol 2022, 2, 58–77. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of Poly(Alkylene Succinate) Biodegradable Polyesters, Part II: Mathematical Modelling of the Polycondensation Reaction. Polymer 2008, 49, 3677–3685. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal Degradation Mechanism of Poly (Ethylene Succinate) and Poly (Butylene Succinate): Comparative Study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar] [CrossRef]
- Bikiaris, D.; Prinos, J.; Panayiotou, C. Effect of EAA and Starch on the Thermooxidative Degradation of LDPE. Polym. Degrad. Stab. 1997, 56, 1–9. [Google Scholar] [CrossRef]
- Burgada, F.; Fages, E.; Quiles-Carrillo, L.; Lascano, D.; Ivorra-Martinez, J.; Arrieta, M.P.; Fenollar, O. Upgrading Recycled Polypropylene from Textile Wastes in Wood Plastic Composites with Short Hemp Fiber. Polymers 2021, 13, 1248. [Google Scholar] [CrossRef]
- Dolçà, C.; Fages, E.; Gonga, E.; Garcia-sanoguera, D.; Balart, R.; Quiles-carrillo, L. The Effect of Varying the Amount of Short Hemp Fibers on Mechanical and Thermal Properties of Wood–Plastic Composites from Biobased Polyethylene Processed by Injection Molding. Polymers 2022, 14, 138. [Google Scholar] [CrossRef]
- Väisänen, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of Hemp Fibers (Cannabis Sativa L.) for Composite Applications. Ind. Crops Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
- Xanthopoulou, E.; Chrysafi, I.; Polychronidis, P.; Zamboulis, A.; Bikiaris, D.N. Evaluation of Eco-Friendly Hemp-Fiber-Reinforced Recycled HDPE Composites. J. Compos. Sci. 2023, 7, 138. [Google Scholar] [CrossRef]
- Marcuello, C.; Chabbert, B.; Berzin, F.; Bercu, N.B.; Molinari, M. Influence of Surface Chemistry of Fiber and Lignocellulosic Materials on Adhesion Properties with Polybutylene Succinate at Nanoscale. Materials 2023, 16, 2440. [Google Scholar] [CrossRef]
- Lostao, A.; Lim, K.S.; Pallarés, M.C.; Ptak, A.; Marcuello, C. Recent Advances in Sensing the Inter-Biomolecular Interactions at the Nanoscale—A Comprehensive Review of AFM-Based Force Spectroscopy. Int. J. Biol. Macromol. 2023, 238, 124089. [Google Scholar] [CrossRef] [PubMed]
- Dolza, C.; Gonga, E.; Fages, E.; Tejada-Oliveros, R.; Balart, R.; Quiles-Carrillo, L. Green Composites from Partially Bio-Based Poly(Butylene Succinate-Co-Adipate)-PBSA and Short Hemp Fibers with Itaconic Acid-Derived Compatibilizers and Plasticizers. Polymers 2022, 14, 1968. [Google Scholar] [CrossRef] [PubMed]
- Tanasă, F.; Zănoagă, M.; Teacă, C.A.; Nechifor, M.; Shahzad, A. Modified Hemp Fibers Intended for Fiber-Reinforced Polymer Composites Used in Structural Applications—A Review. I. Methods of Modification. Polym. Compos. 2020, 41, 5–31. [Google Scholar] [CrossRef]
- Ashori, A. Wood-Plastic Composites as Promising Green-Composites for Automotive Industries! Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial Hemp Fibers: An Overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef]
- Scarponi, C.; Messano, M. Comparative Evaluation between E-Glass and Hemp Fiber Composites Application in Rotorcraft Interiors. Compos. B Eng. 2015, 69, 542–549. [Google Scholar] [CrossRef]
- Sathish, T.; Palani, K.; Natrayan, L.; Merneedi, A.; de Poures, M.V.; Singaravelu, D.K. Synthesis and Characterization of Polypropylene/Ramie Fiber with Hemp Fiber and Coir Fiber Natural Biopolymer Composite for Biomedical Application. Int. J. Polym. Sci. 2021, 2021, 2462873. [Google Scholar] [CrossRef]
- Ticoalu, A.; Aravinthan, T.; Cardona, F. A Review of Current Development in Natural Fiber Composites in Automotive Applications. Appl. Mech. Mater. 2010, 564, 3–7. [Google Scholar] [CrossRef]
- Awwad, E.; Mabsout, M.; Hamad, B.; Farran, M.T.; Khatib, H. Studies on Fiber-Reinforced Concrete Using Industrial Hemp Fibers. Constr. Build. Mater. 2012, 35, 710–717. [Google Scholar] [CrossRef]
- Abu-Saleem, M.; Zhuge, Y.; Hassanli, R.; Ellis, M.; Rahman, M.; Levett, P. Evaluation of Concrete Performance with Different Types of Recycled Plastic Waste for Kerb Application. Constr. Build. Mater. 2021, 293, 123477. [Google Scholar] [CrossRef]
- Tran Le, A.D.; Maalouf, C.; Mai, T.H.; Wurtz, E.; Collet, F. Transient Hygrothermal Behaviour of a Hemp Concrete Building Envelope. Energy Build. 2010, 42, 1797–1806. [Google Scholar] [CrossRef]
- Shahzad, A. Hemp Fiber and Its Composites—A Review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, Y.; Deng, J.; Rodrigue, D. Effect of Fiber Treatment on the Water Absorption and Mechanical Properties of Hemp Fiber/Polyethylene Composites. J. Appl. Polym. Sci. 2013, 127, 942–949. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Fierascu, R.C.; Gabor, A.R.; Nicolae, C.A. Effect of Hemp Fiber Length on the Mechanical and Thermal Properties of Polypropylene/SEBS/Hemp Fiber Composites. J. Mater. Res. Technol. 2020, 9, 10768–10781. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Zhou, W.; Zhi, E.; Zhang, W.; Ji, J. Graphene-Reinforced Biodegradable Poly(Ethylene Succinate) Nanocomposites Prepared by In Situ Polymerization. J. Appl. Polym. Sci. 2013, 130, 3212–3220. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Chrissafis, K.; Paraskevopoulos, K.M.; Triantafyllidis, K.S.; Antonakou, E.V. Investigation of Thermal Degradation Mechanism of an Aliphatic Polyester Using Pyrolysis-Gas Chromatography-Mass Spectrometry and a Kinetic Study of the Effect of the Amount of Polymerisation Catalyst. Polym. Degrad. Stab. 2007, 92, 525–536. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Effect of Molecular Weight on Thermal Degradation Mechanism of the Biodegradable Polyester Poly(Ethylene Succinate). Thermochim. Acta 2006, 440, 166–175. [Google Scholar] [CrossRef]
- Klonos, P.A.; Papadopoulos, L.; Kasimatis, M.; Iatrou, H.; Kyritsis, A.; Bikiaris, D.N. Synthesis, Crystallization, Structure Memory Effects, and Molecular Dynamics of Biobased and Renewable Poly (n-Alkylene Succinate)s with n from 2 to 10. Macromolecules 2021, 54, 1106–1119. [Google Scholar] [CrossRef]
- Asimakidou, T.; Chrissafis, K. Thermal Behavior and Pyrolysis Kinetics of Olive Stone Residue. J. Therm. Anal. Calorim. 2022, 147, 9045–9054. [Google Scholar] [CrossRef]
- Hamadache, H.; Djidjelli, H.; Boukerrou, A.; Kaci, M.; José Antonio, J.R.; Martín-Martínez, J.M. Different Compatibility Approaches to Improve the Thermal and Mechanical Properties of EVA/Starch Composites. Polym. Compos. 2019, 40, 3242–3253. [Google Scholar] [CrossRef]
- Eszer, N.H.; Ishak, Z.A.M. Effect of Compatibilizer on Morphological, Thermal and Mechanical Properties of Starch-Grafted-Polypropylene/Kenaf Fibers Composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012017. [Google Scholar] [CrossRef]
- Zoukrami, F.; Haddaoui, N.; Sclavons, M.; Devaux, J.; Vanzeveren, C. Rheological Properties and Thermal Stability of Compatibilized Polypropylene/Untreated Silica Composites Prepared by Water Injection Extrusion Process. Polym. Bull. 2018, 75, 5551–5566. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Kim, H.J.; Yang, H.S. Thermal Properties of Bio-Flour-Filled Polyolefin Composites with Different Compatibilizing Agent Type and Content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. Thermal Degradation Kinetics and Decomposition Mechanism of Polyesters Based on 2,5-Furandicarboxylic Acid and Low Molecular Weight Aliphatic Diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. Thermochimica Acta ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetic Study of Stabilizing Effect of Oxygen on Thermal Degradation of Poly(Methyl Methacrylate). J. Phys. Chem. B 1999, 103, 8087–8092. [Google Scholar] [CrossRef]
- Chrissafis, K. Detail Kinetic Analysis of the Thermal Decomposition of PLA with Oxidized Multi-Walled Carbon Nanotubes. Thermochim. Acta 2010, 511, 163–167. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the Integral Isoconversional Method to Account for Variation in the Activation Energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of Activation Energy of Thermally Stimulated Solid-State Reactions under Arbitrary Variation of Temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A Review on Thermal Decomposition and Combustion of Thermoplastic Polyesters. Polym. Adv. Technol. 2004, 15, 691–700. [Google Scholar] [CrossRef]
- Nguyen, S.; Yu, G.; Marchessault, R.H. Thermal Degradation of Poly(3-Hydroxyalkanoates): Preparation of Well-Defined Oligomers. Biomacromolecules 2002, 3, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.L.; Vyazovkin, S. Thermal Properties and Degradation Behavior of Linear and Branched Poly(L-Lactide)s and Poly(L-Lactide-Co-Glycolide)S. Macromol. Chem. Phys. 2012, 213, 924–936. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Achilias, D.S.; Vaimakis, T.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal Degradation of Biobased Polyesters: Kinetics and Decomposition Mechanism of Polyesters from 2,5-Furandicarboxylic Acid and Long-Chain Aliphatic Diols. J. Anal. Appl. Pyrolysis 2016, 117, 162–175. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D. Thermal Degradation Kinetics and Decomposition Mechanism of Two New Aliphatic Biodegradable Polyesters Poly(Propylene Glutarate) and Poly(Propylene Suberate). Thermochim. Acta 2010, 505, 59–68. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Decomposition Mechanism of Polyesters Based on 2,5-Furandicarboxylic Acid and Aliphatic Diols with Medium and Long Chain Methylene Groups. Polym. Degrad. Stab. 2016, 132, 127–136. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Carroccio, S. Thermo-Oxidative Processes in Biodegradable Poly(Butylene Succinate). Polym. Degrad. Stab. 2009, 94, 1825–1838. [Google Scholar] [CrossRef]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A.S. Targeted Pre-Treatment of Hemp Bast Fibres for Optimal Performance in Biocomposite Materials: A Review. Ind. Crops Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef]
- Zimniewska, M. Hemp Fibre Properties and Processing Target Textile: A Review. Materials 2022, 15, 1901. [Google Scholar] [CrossRef]

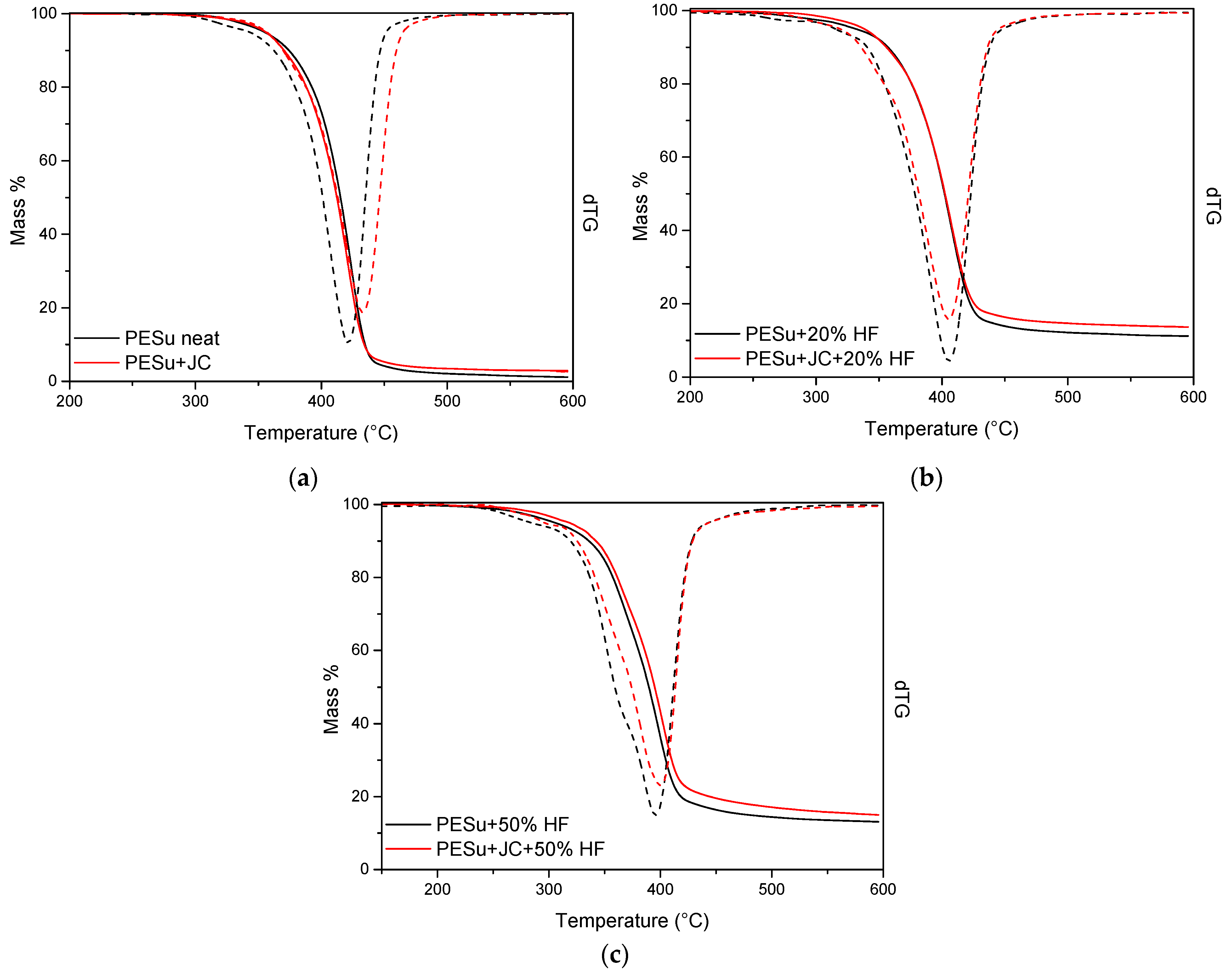




| Sample | Td1 (°C) | Td2,max (°C) | Remaining Mass % |
|---|---|---|---|
| PESu + JC | - | 433.6 | 2.7 |
| PESu + JC + 10% HF | - | 408.2 | 10.1 |
| PESu + JC + 20% HF | 299.8 | 405.5 | 13.7 |
| PESu + JC + 50% HF | 297.3 | 400.2 | 14.9 |
| PESu + JC + 75% HF | 289.9 | 357.5 | 19.8 |
| Sample | Step | Mechanism | Eα (kJ/mol) | logA (s−1) | logKcat | React. Order n | R2 |
|---|---|---|---|---|---|---|---|
| PESu | 1st | Cn | 107 | 6 | 0.3 | 0.6 | 0.99994 |
| 2nd | Cn | 170 | 10.7 | 1.2 | 2.1 | ||
| PESu + JC | 1st | Cn | 75 | 3.6 | 0.1 | 0.3 | 0.99994 |
| 2nd | Cn | 114 | 6.4 | 0.8 | 0.8 | ||
| 3rd | Cn | 172 | 10.5 | 1.9 | 3.2 | ||
| PESu + 20% HF | 1st | Cn | 102 | 5.6 | 0.01 | 0.6 | 0.99992 |
| 2nd | Cn | 180 | 12.7 | 0.4 | 3.1 | ||
| PESu + JC + 20% HF | 1st | Cn | 65 | 3.2 | 0.01 | 1.1 | 0.99994 |
| 2nd | Cn | 123 | 0.7 | 0.01 | 0.7 | ||
| 3rd | Cn | 246 | 17.6 | 0.9 | 4.2 |
| Rt (min) | Sample Name | Mw (amu) | Assigned Compound | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PESu + JC | PESu neat | PESu + JC + 10% HF | PESu + JC + 20%HF | PESu + 20% HF | PESu + JC + 50% HF | PESu + 50% HF | PESu + JC + 75% HF | |||
| Relative Intensity (%) | ||||||||||
| 0.6 | 5.73 | - | 8.24 | 2.43 | 8.02 | n.d. | n.d. | 38.3 | 40–44 | Carbox dioxide |
| 1.7 | 21.74 | 27.46 | 19.42 | 1.28 | 20.36 | 22.95 | 25.98 | 24.79 | 72 | 2-propenoic acid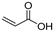 |
| 2.0 | n.d. | n.d. | n.d. | n.d. | n.d. | 8.82 | 3.56 | 16.27 | 88 | 1-hydroxybutan-2-one |
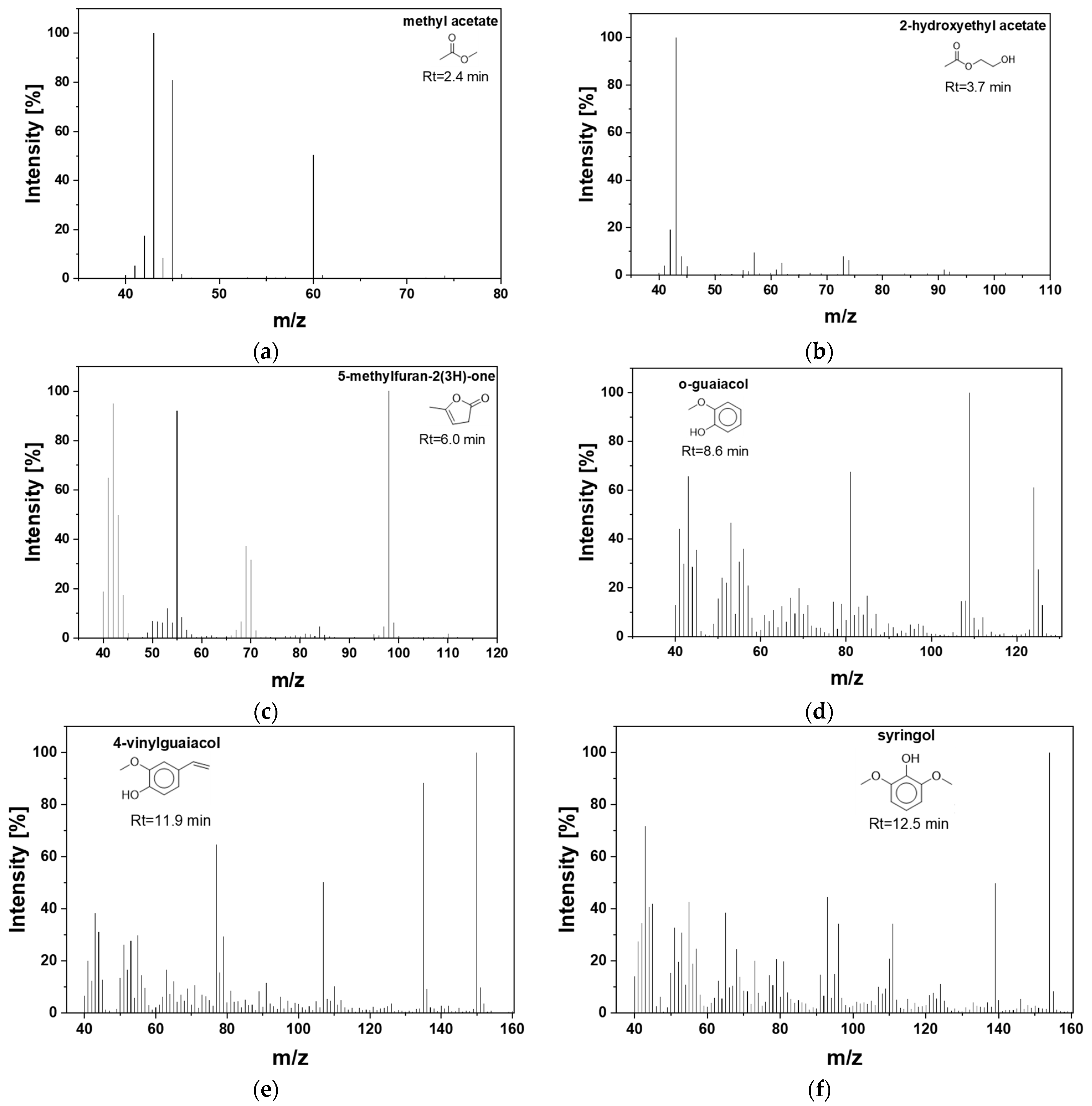
| 2.4 | n.d. | n.d. | n.d. | 2.21 | n.d. | 11.32 | 3.8 | 41.08 | 74 | methyl acetate |
| 3.7 | n.d. | n.d. | n.d. | n.d. | n.d. | 4.24 | 2.01 | 15.61 | 102 | 2-hydroxyethyl acetate |
| 4.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6.18 | 108 | p-cresol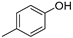 |
| 6.0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 12.5 | 98 | 5-methylfuran-2(3H)-one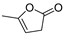 |
| 6.3 | 12.3 | 12.57 | 6.03 | 1.21 | 2.98 | 2.71 | 2.32 | 3.87 | 100 | Propanoic acid, 2-hydroxyethyl ester |
| 8.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 7.85 | 124 | 2-methoxy-phenol (o-Guaiacol)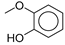 |
| 9.2 | 21.84 | 18.43 | 6.01 | 16.72 | 5.43 | 7.11 | 5.24 | 5.28 | 114 | Succinic anhydride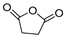 |
| 10.7 | 4.57 | 5.36 | 3.96 | 4.36 | 3.44 | 7.32 | 3.62 | 14.23 | 143 | 4-oxo-4-(vinyloxy)butanoic acid |
| 11.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 7.06 | 150 | 4-Vinylguaiacol |
| 12.5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 7.01 | 154 | 2,6-dimethoxy-phenol (syringol) 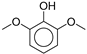 |
| 13.1 | 8.31 | 7.29 | 6.38 | 9.74 | 2.17 | 16.48 | 2.24 | 8.52 | 158 | 4-(allyloxy)-4-oxobutanoic acid |
| 13.3 | 41.35 | 40.92 | 23.08 | 59.08 | 14.96 | 1.58 | 17.89 | 4.27 | 160 | 4-(2-hydroxyethoxy)-4-oxobutanoic acid |
| 13.6 | 5.01 | 7.6 | 2.37 | 3.81 | 1.6 | 1.44 | n.d. | 4.11 | 160 | 4-oxo-4-(2-oxoethoxy)butanoic acid |
| 15.5 | 4.52 | 3.27 | 1.59 | 2.63 | n.d. | 1.8 | n.d. | 7.89 | 172 | Butanedioic acid, diethyl ester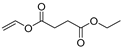 |
| 15.9 | 5.80 | 4.87 | 1.07 | 8.76 | 1.12 | 1.97 | 1.42 | 3.83 | 186 | 2-oxoethyl vinyl succinate |
| 16.9 | 22.59 | 27.1 | 21.4 | 38.2 | 24.66 | 46.88 | 32.84 | 74.19 | 174 | ethane-1,2-diyl dipropionate |
| 17.6 | 5.31 | 6.60 | 4.96 | 11.53 | 6.94 | 9.38 | 7.61 | 13.78 | 189 | 2-hydroxyethyl vinyl succinate |
| 18.7 | 42.02 | 37.02 | 22.28 | 49.68 | 12.27 | 10.32 | 10.4 | 4.87 | 201 | Allyl (2-hydroxyethyl) succinate |
| 20.3 | 3.92 | 3.00 | 1.79 | 5.81 | n.d. | 1.58 | n.d. | 2.7 | 258 | 2-(acryloyloxy)ethyl (2-hydroxyethyl) succinate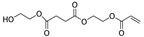 |
| 20.6 | 5.01 | 3.44 | 2.01 | 7.81 | n.d. | 1.37 | n.d. | 2.65 | 202 | 2-(propionyloxy)ethyl 4-oxobutanoate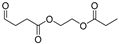 |
| 21.5 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 65.12 | 262 | 4,4′-(ethane-1,2-diylbis(oxy))bis(4-oxobutanoic acid)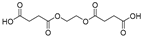 |
| 23.0 | 7.11 | 23.96 | 9.12 | 13.13 | 6.7 | 12.15 | 13 | 5.8 | 289 | 4-oxo-4-(2-((4-oxo-4-(vinyloxy)butanoyl)oxy)ethoxy)butanoic acid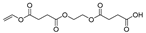 |
| 23.7 | 20.59 | 26.4 | 12.73 | 25.19 | 6.29 | 8.97 | 7.47 | 5.96 | 288 | 2-((4-oxobutanoyl)oxy)ethyl (2-oxoethyl) succinate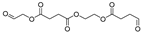 |
| 24.1 | 4.78 | 3.62 | 3.89 | 15.55 | n.d. | 6.17 | 1.5 | 10.59 | 312 | O,O′-(ethane-1,2-diyl) divinyl disuccinate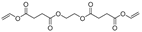 |
| 29.1 | 27.1 | 30.7 | 34.38 | 27.85 | 24.97 | 32.67 | 22.03 | 37.68 | 333 | 2-((4-(2-hydroxyethoxy)-4-oxobutanoyl)oxy)ethyl vinyl succinate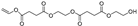 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysafi, I.; Ainali, N.M.; Xanthopoulou, E.; Zamboulis, A.; Bikiaris, D.N. Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Ethylene Succinate)/Hemp Fiber Composites. J. Compos. Sci. 2023, 7, 216. https://doi.org/10.3390/jcs7060216
Chrysafi I, Ainali NM, Xanthopoulou E, Zamboulis A, Bikiaris DN. Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Ethylene Succinate)/Hemp Fiber Composites. Journal of Composites Science. 2023; 7(6):216. https://doi.org/10.3390/jcs7060216
Chicago/Turabian StyleChrysafi, Iouliana, Nina Maria Ainali, Eleftheria Xanthopoulou, Alexandra Zamboulis, and Dimitrios N. Bikiaris. 2023. "Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Ethylene Succinate)/Hemp Fiber Composites" Journal of Composites Science 7, no. 6: 216. https://doi.org/10.3390/jcs7060216
APA StyleChrysafi, I., Ainali, N. M., Xanthopoulou, E., Zamboulis, A., & Bikiaris, D. N. (2023). Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Ethylene Succinate)/Hemp Fiber Composites. Journal of Composites Science, 7(6), 216. https://doi.org/10.3390/jcs7060216







