Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement
Abstract
1. Introduction
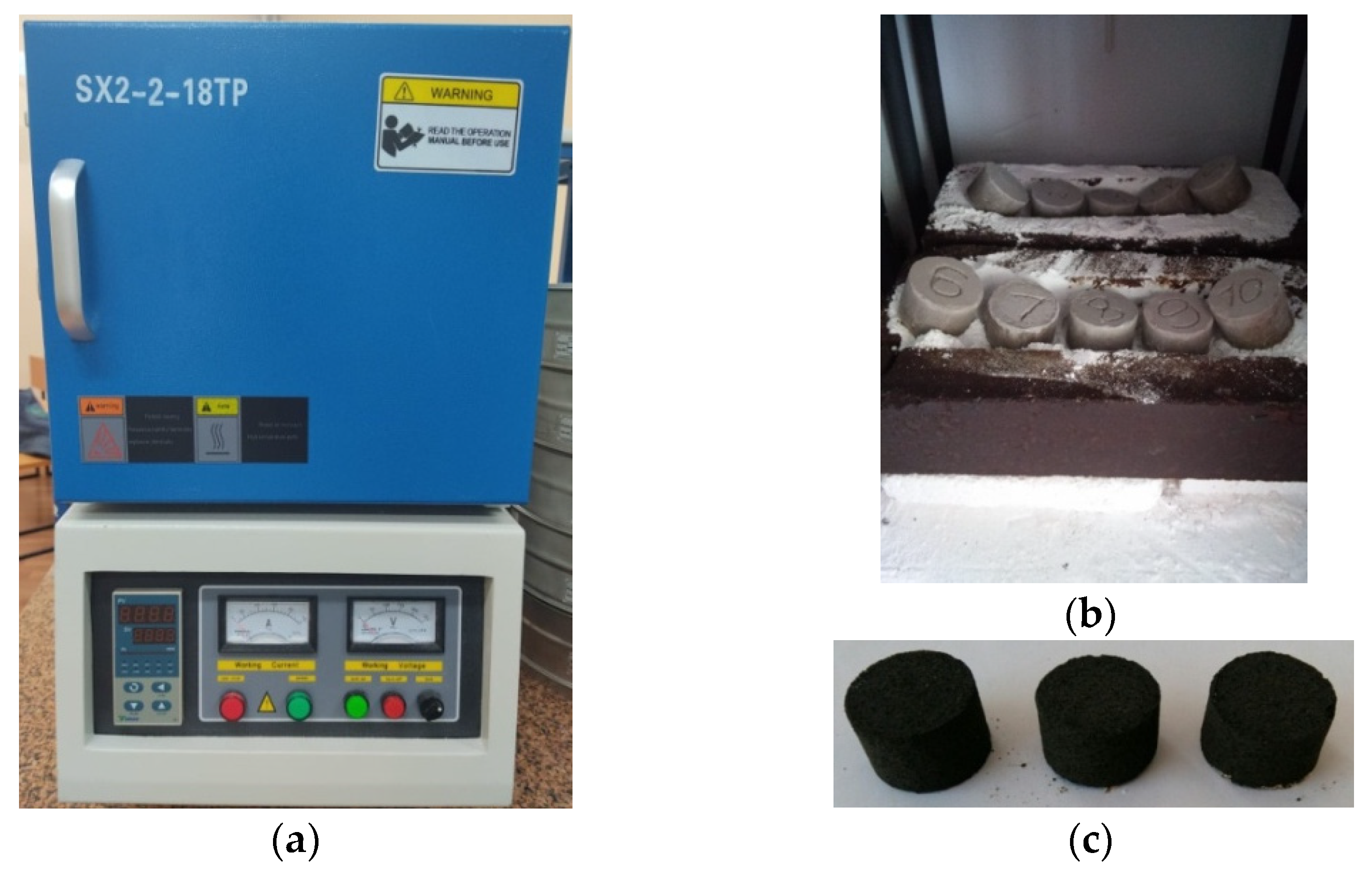
2. Materials and Research Methods
2.1. Limestone of the Sastobe Deposit
2.2. Coal Mining Waste
2.3. Tefritobasalt
2.4. Lead Slag
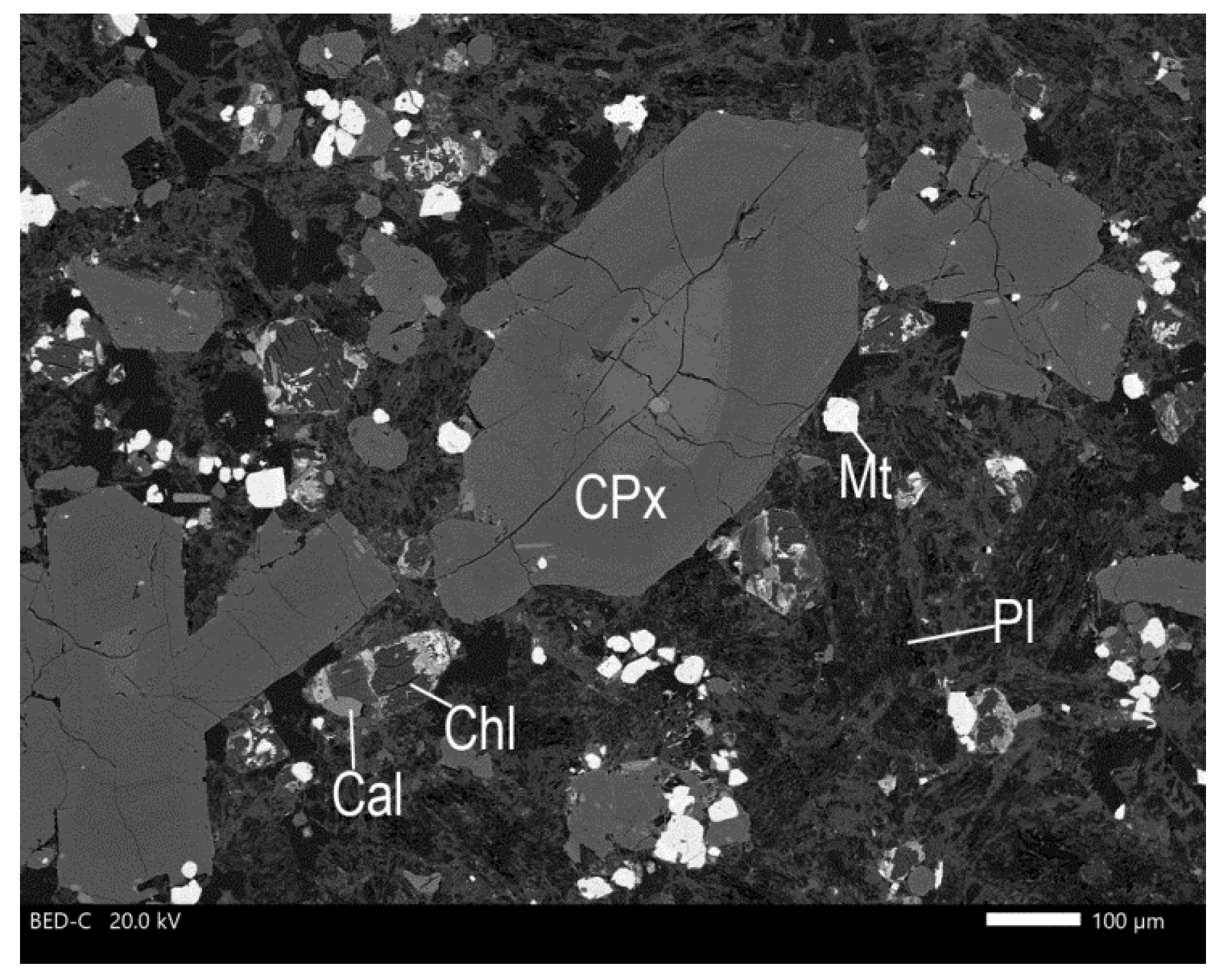
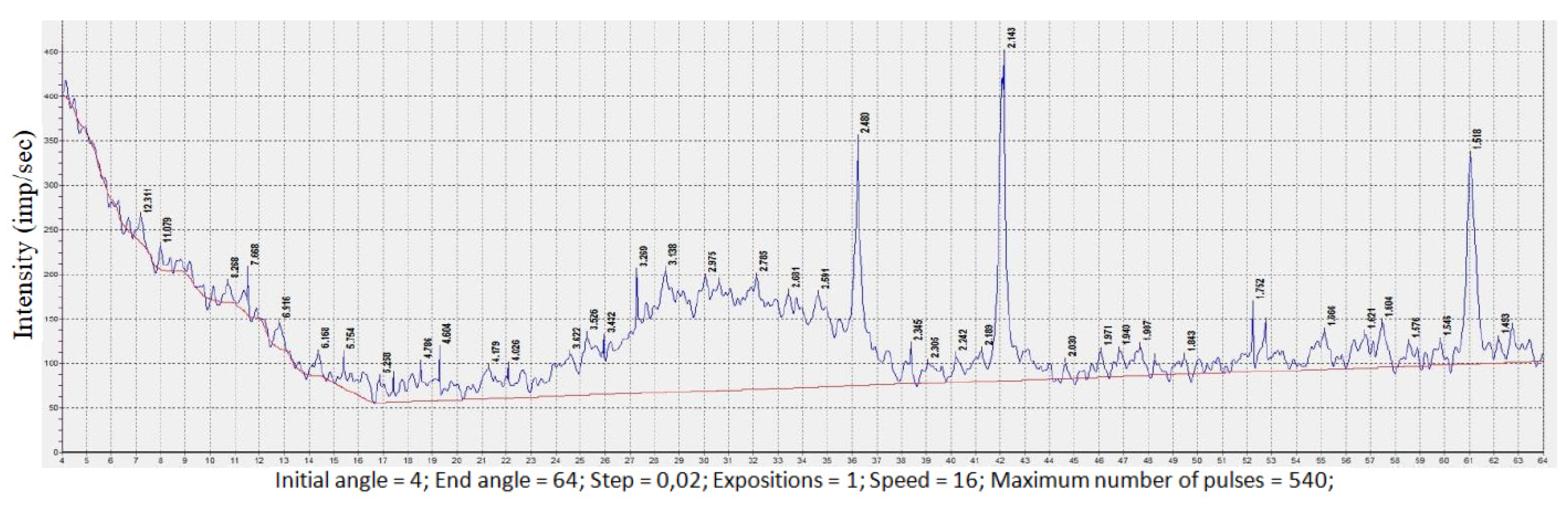
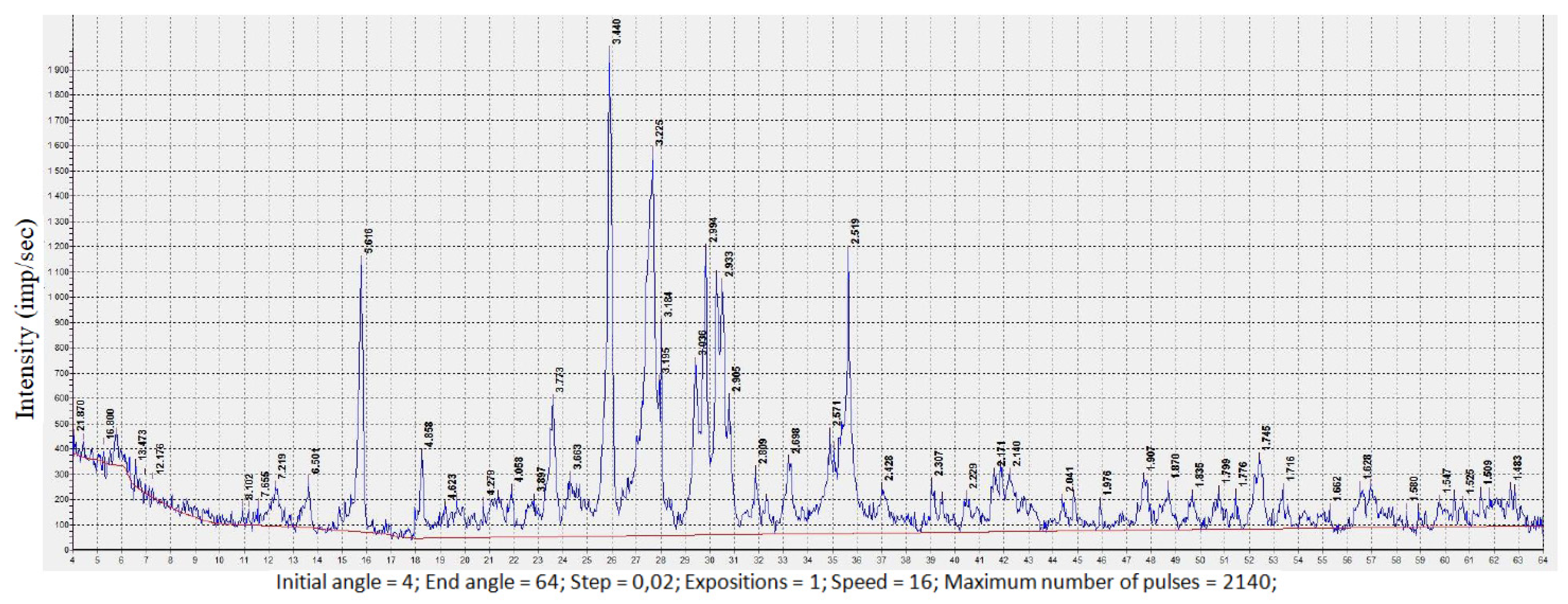
3. Results and Discussion
4. Conclusions
- The conducted research shows the possibility of replacing traditional raw materials with man-made industrial waste such that economically beneficial phenomena, including resource conservation and utilization of man-made waste, are possible, while reducing the anthropogenic impact on the region.
- In the studied initial experimental compositions, the replacement of the clay- and iron-containing component with tefritobasalt and lead slag led to a decrease in the melting temperature of the mixture by 100 °C relative to traditional compositions and is environmentally and economically feasible.
- In the samples obtained from the four-component raw material mixture “Limestone—tefritobasalt—coal mining waste—lead slag”, it is clear that the synthesized clinker has a full-crystalline structure with a content of 57.88% alite and 18.82% belite.
- According to the calculation results, SC = 0.94, silicate modulus n = 2.02, and alumina modulus p = 0.95. The theoretical specific consumption of raw materials is 1481 t/t of clinker, which is approximately 70 kg lower than in traditional mixtures. The content of non-traditional components in total was 24.69%.
- According to the results of chemical analysis, the content of free CaO in the clinker was 0.2%, which meets the requirements of GOST. The quality of the synthesized clinker was high, the minerals were formed correctly, and the size of the alite crystals was 20–50 microns.
- The strength of the obtained Portland cement after 28 days under compression was 41.8 MPa, which corresponds to the cement grade of strength M400 according to GOST 10178-85 or strength class 42.5 according to GOST 31108-2016.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhanikulov, N.; Khudyakova, T.; Taimassov, B.; Sarsenbayev, B.; Dauletiarov, M.; Kolesnikov, A.; Karshygayev, R. Receiving Portland Cement from Technogenic Raw Materials of South Kazakhstan. Eurasian Chem.-Technol. J. 2019, 21, 333. [Google Scholar] [CrossRef]
- Kolesnikova, O.; Vasilyeva, N.; Kolesnikov, A.; Zolkin, A. Optimization of raw mix using technogenic waste to produce cement clinker. Min. Inf. Anal. Bull 2022, 60, 103–115. [Google Scholar] [CrossRef]
- Zhantasov, M.K.; Bimbetova, G.Z.; Kolesnikov, A.S.; Sadyrbayeva, A.S.; Orynbasarov, A.K.; Kutzhanova, A.N.; Turemuratov, R.S.; Botabaev, N.E.; Zhantasova, D. Examination of optimal parameters of oxy-ethylation of fatty acids with a view to obtaining demulsifiers for deliquefaction in the system of skimming and treatment of oil: A method to obtain demulsifier from fatty acids. Chem. Today 2016, 34, 72–77. [Google Scholar]
- Zhanikulov, N.N.; Kolesnikov, A.S.; Taimasov, B.T.; Zhakipbayev, B.Y.; Shal, A.L. Influence of industrial waste on the structure of environmentally friendly cement clinker. Complex Use Miner. Resour. 2022, 4, 84–91. [Google Scholar] [CrossRef]
- Zhakipbaev, B.Y.; Zhanikulov, N.N.; Kolesnikova, O.G.; Akhmetova, E.K.; Kuraev, R.M.; Shal, A.L. Review of technogenic waste and methods of its processing for the purpose of complex utilization of tailings from the enrichment of non-ferrous metal ores as a component of the raw materials mixture in the production of cement clinker. Rasayan J. Chem. 2021, 14, 997–1005. [Google Scholar]
- Taimasov, B.T.; Borisov, I.N.; Dzhanmuldaeva, Z.K.; Dauletiarov, M.S. Research on obtaining low energy cements from technogenic raw materials. J. Chem. Technol. Metall. 2020, 55, 814–823. [Google Scholar]
- Hassaan, M. Basalt rock as an alternative raw material in Portland cement manufacture. Mater. Lett. 2001, 50, 172–178. [Google Scholar] [CrossRef]
- Siziakova, E.; Ivanov, P.; Boikov, A. Hydrocarboaliminate calcium application in aluminum processing for production of special cement brands. J. Phys. Conf. Ser. 2018, 1118, 012018. [Google Scholar] [CrossRef]
- Abd El-Hafiz, N.A.; Abd El-Moghny, M.W.; El-Desoky, H.M.; Afifi, A.A. Characterization and technological behavior of basalt raw materials for Portland cement clinker production. IJISET-Int. J. Innov. Sci. Eng. Technol. 2015, 2, 1–22. [Google Scholar]
- Taimasov, B.T. Chemical Technology of Binding Materials; Evero: Almaty, Kazakhstan, 2015. [Google Scholar]
- Gorshkov, V.S.; Aleksandrov, S.E.; Ivashchenko, S.I.; Gorshkova, I.V. Complex Processing and Use of Metallurgical Slags in Construction; Stroyizdat: Moscow, Russia, 1985. [Google Scholar]
- Klassen, V.K.; Borisov, I.N.; Manuilov, V.E. Technogenic Materials in Cement Production, Monograph; Publishing House of BSTU: Belgorod, Russia, 2008. [Google Scholar]
- Taimasov, B.T.; Khudyakova, T.M.; Zhanikulov, N.N. Integrated Use of Natural and Technogenic Raw Materials in the Production of Low-Energy Cements; Auezov SKSU: Shymkent, Kazakhstan, 2017. [Google Scholar]
- Reed, S.D. b. Electron Probe Microanalysis and Scanning Electron Microscopy in Geology; Technosphere: Moscow, Russia, 2008. [Google Scholar]
- Bishimbaev, V.K.; Rusnak, V.V.; Egorov, Y.V. Mineral and Raw Material and Technological Base of the South-Kazakhstan Cluster of Building and Silicate Materials; Rarity: Almaty, Kazakhstan, 2009. [Google Scholar]
- Kulikova, E.Y.; Balovtsev, S.V.; Skopintseva, O.V. Complex estimation of geotechnical risks in mine and underground construction. Sustain. Develop. Mount.Territ. 2023, 15, 7–16. [Google Scholar] [CrossRef]
- Taimasov, B.T.; Sarsenbayev, B.K.; Khudyakova, T.M.; Kolesnikov, A.S.; Zhanikulov, N.N. Development and testing of low-energy-intensive technology of receiving sulphate-resistant and road portlandcement. Eurasian Chem.-Technol. J. 2017, 19, 347–355. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Serikbaev, B.E.; Zolkin, A.L.; Kenzhibaeva, G.S.; Isaev, G.I.; Botabaev, N.E.; Shapalov, S.K.; Kolesnikova, O.G.; Iztleuov, G.M.; Suigenbayeva, A.Z.; et al. Processing of Non-Ferrous Metallurgy Waste Slag for its Complex Recovery as a Secondary Mineral Raw Material. Refract. Ind. Ceram. 2021, 62, 375–380. [Google Scholar] [CrossRef]
- Kolesnikova, O.; Syrlybekkyzy, S.; Fediuk, R.; Yerzhanov, A.; Nadirov, R.; Utelbayeva, A.; Agabekova, A.; Latypova, M.; Chepelyan, L.; Volokitina, I.; et al. Thermodynamic Simulation of Environmental and Population Protection by Utilization of Technogenic Tailings of Enrichment. Materials 2022, 15, 6980. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Groves, D.I. Mineral Resource Reviews. In Potassic Igneous Rocks and Associated Gold-Copper Mineralization; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-92978-1. [Google Scholar]
- Donayev, A.; Kolesnikov, A.; Shapalov, S.; Sapargaliyeva, B.; Ivakhniyuk, G. Studies of waste from the mining and metallurgical industry, with the determination of its impact on the life of the population. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2022, 4, 55–68. [Google Scholar] [CrossRef]
- Utelbayeva, A.; Kolesnikov, A.; Aldiyarov, Z.; Dossybekov, S.; Esimov, E.; Duissenbekov, B.; Fediuk, R.; Vatin, N.I.; Yermakhanov, M.; Mussayeva, S. Experimental Analysis of the Stress State of a Prestressed Cylindrical Shell with Various Structural Parameters. Materials 2022, 15, 4996. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Sapargaliyeva, B.; Bychkov, A.Y.; Alferyeva, Y.O.; Syrlybekkyzy, S.; Altybaeva, Z.; Nurshakhanova, L.; Seidaliyeva, L.; Suleimenova, B.; Zhidebayeva, A.; et al. Thermodynamic modeling of the formation of the main minerals of cement clinker and zinc fumes in the processing of toxic technogenic waste of the metallurgical industry. Rasayan J. Chem. 2022, 15, 2181–2187. [Google Scholar] [CrossRef]
- Baibatsha, A.B. Geology of Mineral Deposits; KazNTU: Almaty, Kazakhstan, 2008. [Google Scholar]
- Seignez, N.; Bulteel, D.; Damidot, D.; Gauthier, A.; Potdevin, J.L. Weathering of metallurgical slag heaps: Multi-experimental approach of the chemical behaviours of lead and zinc. WIT Trans. Ecol. Environ. 2006, 92, 31–40. [Google Scholar]
- Trubaev, P. Methodological Guide to the Application of the Rocs Program; V.G. Shukhov BSTU: Belgorod, Russia, 2006. [Google Scholar]
- Butt, Y.M.; Timashev, V.V. Workshop on Chemical Technology of Binders; Higher School: Moscow, Russia, 1973. [Google Scholar]
- Naizabekov, A.; Volokitina, I.; Kolesnikov, A. Changes In Microstructure And Properties Of Austenitic Steel AISi 316 during High-Pressure Torsion. J. Chem. Technol. Metallur. 2022, 57, 809–815. [Google Scholar]
- Kuznetsova, T.V.; Samchenko, S.V. Microscopy of Cement Production Materials; MIKHiS: Moscow, Russia, 2007. [Google Scholar]
- Fediuk, R. High-strength fibrous concrete of Russian Far East natural materials. IOP Conf. Ser. Mater. Sci. Eng. 2016, 116, 012020. [Google Scholar] [CrossRef]
- Ponzi, G.G.D.; dos Santos, V.H.J.M.; Martel, R.B.; Pontin, D.; Stepanha, A.S.D.G.E.; Schütz, M.K.; Menezes, S.C.; Einloft, S.M.; Vecchia, F.D. Basalt powder as a supplementary cementitious material in cement paste for CCS wells: Chemical and mechanical resistance of cement formulations for CO2 geological storage sites. Int. J. Greenh. Gas Control. 2021, 109, 103337. [Google Scholar] [CrossRef]
- Bolio-Arcero, H.; Glasser, F.P. Zinc oxide in cement clinkering: Part 1 systems CaO–ZnO–Al2O3 and CaO–ZnO–Fe2O3. Adv. Cem. Res. 1998, 25, 10. [Google Scholar]
- Gineys, N. Influence de la Teneuren Elements Métalliques du Clinker sur les Proprieties Techniques et Environnementales du Ciment Portland. Ph.D. Thesis, Université Lille Nord de France, Lille, France, 2011. [Google Scholar]
- Matusiewicz, A.; Bochenek, A.; Szelag, H.; Kurdowski, W. Pewne zagadnienia zwiazane z podwyzszona zawartoscia cynku w klinkierze i w produkowanym z niego cemencie. Cem. Wapno Beton 2011, 78, 332–341. [Google Scholar]
- Kolesnikov, A.; Fediuk, R.; Amran, M.; Klyuev, S.; Klyuev, A.; Volokitina, I.; Naukenova, A.; Shapalov, S.; Utelbayeva, A.; Kolesnikova, O.; et al. Modeling of Non-Ferrous Metallurgy Waste Disposal with the Production of Iron Silicides and Zinc Distillation. Materials 2022, 15, 2542. [Google Scholar] [CrossRef] [PubMed]
- Auyesbek, S.T.; Sarsenbayev, N.B.; Sarsenbayev, B.K.; Khudyakova, T.M.; Aimenov, Z.T.; Abdiramanova, K.S.; Aubakirova, T.S.; Sauganova, G.R.; Kolesnikova, O.G.; Karshyga, G.O.; et al. Thermal Insulating Materials Based on Magnesium-Containing Technogenic Raw Materials. Rasayan J. Chem. 2023, 16, 413–421. [Google Scholar] [CrossRef]
- Sergeeva, I.V.; Botabaev, N.E.; Al’Zhanova, A.Z.; Ashirbaev, K.A. Thermodynamic simulation of chemical and phase transformations in the system of oxidized manganese ore—Carbon. Izv. Ferr. Metall. 2017, 60, 759–765. [Google Scholar]
- Auyesbek, S.; Sarsenbayev, N.; Abduova, A.; Sarsenbayev, B.; Uderbayev, S.; Aimenov, Z.; Kenzhaliyeva, G.; Akishev, U.; Aubakirova, T.; Sauganova, G.; et al. Man-Made Raw Materials for the Production of Composite Silicate Materials Using Energy-Saving Technology. J. Compos. Sci. 2023, 7, 124. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Fediuk, R.; Kolesnikova, O.; Zhanikulov, N.; Zhakipbayev, B.; Kuraev, R.; Akhmetova, E.; Shal, A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials 2022, 15, 324. [Google Scholar] [CrossRef]
- Zhangabay, N.; Suleimenov, U.; Utelbayeva, A.; Kolesnikov, A.; Baibolov, K.; Imanaliyev, K.; Moldagaliyev, A.; Karshyga, G.; Duissenbekov, B.; Fediuk, R.; et al. Analysis of a Stress-Strain State of a Cylindrical Tank Wall Vertical Field Joint Zone. Buildings 2022, 12, 1445. [Google Scholar] [CrossRef]
- Naizabekov, A.B.; Kolesnikov, A.S.; Latypova, M.A.; Fedorova, T.D.; Mamitova, A.D. Current Trends to Obtain Metals and Alloys with Ultrafine-Grained Structure. Progr. Phys. Met. 2022, 23, 629–657. [Google Scholar]
- Kolesnikov, A.S.; Zhanikulov, N.N.; Syrlybekkyzy, S.; Kolesnikova, O.G.; Shal, A.L. Utilization of Waste from the Enrichment of Non-Ferrous Metal Ores as Secondary Mineral Raw Materials in the Production of Cement Clinker. Ecol. Ind. Russ. 2023, 27, 19–23. [Google Scholar]
- Efremova, S.V. Scientific and technical solutions to the problem of utilization of waste from plant- and mineral-based industries. Russ. J. Gen. Chem. 2012, 82, 963–968. [Google Scholar] [CrossRef]
- Volokitina, I.E. Evolution of the Microstructure and Mechanical Properties of Copper under ECAP with Intense Cooling. Met. Sci. Heat Treat. 2020, 62, 253–258. [Google Scholar] [CrossRef]
- Volokitina, I.; Vasilyeva, N.; Fediuk, R.; Kolesnikov, A. Hardening of Bimetallic Wires from Secondary Materials Used in the Construction of Power Lines. Materials 2022, 15, 3975. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Zhanikulov, N.; Zhakipbayev, B.; Kolesnikova, O.; Kuraev, R. Thermodynamic modeling of the synthesis of the main minerals of cement clinker from technogenic raw materials. Complex Use Miner. Resour. 2021, 318, 24–34. [Google Scholar] [CrossRef]






| Name | The Chemical Composition, mas. % | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | Cl | Cr2O3 | ZnO | PbO | CuO | Loss of Ignition | Other | Total | |
| Limestone | 3.87 | 1.04 | 0.57 | 52.83 | 0.88 | 0.10 | - | 0.12 | 0.018 | 0.02 | 0.012 | - | - | - | 40.20 | 0.37 | 100.0 |
| Tefrito basalt | 45.54 | 10.70 | 8.53 | 10.66 | 6.95 | 0.20 | 4.04 | 2.50 | 0.91 | 0.017 | 0.007 | - | - | - | 5.37 | 4.57 | 100.0 |
| Coal mining waste | 55.50 | 10.60 | 2.01 | 3.21 | 0.70 | 0.79 | - | 2.35 | 0.38 | - | - | - | - | - | 24.08 | 0.38 | 100.0 |
| Lead slag | 25.94 | 6.44 | 37.25 | 14.71 | 6.15 | 0.04 | 1.24 | 1.36 | 0.35 | 0.006 | 0.063 | 4.34 | 0.52 | 0.94 | 0.65 | - | 100.0 |
| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | L.W.C | Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Limestone Sastobe | 3.87 | 1.04 | 0.57 | 52.83 | 0.88 | 0.10 | 40.71 | - | |||||
| Tefritobasalt Daubaba | 45.54 | 10.70 | 8.53 | 10.66 | 6.95 | 0.20 | 7.92 | 9.50 | |||||
| Coal mining waste from the Lenger mines | 55.50 | 10.60 | 2.01 | 3.21 | 0.70 | 0.79 | 24.08 | 3.11 | |||||
| Lead slag of Yuzhpolimetall JSC plant | 25.94 | 6.44 | 37.25 | 14.71 | 6.15 | 0.04 | 0.10 | 9.37 | |||||
| By component chemical composition of the raw batch | Component content | ||||||||||||
| kg/kg clinker | % | ||||||||||||
| Limestone | 2.91 | 0.78 | 0.43 | 39.78 | 0.66 | 0.075 | 30.65 | - | 1.1160 | 75.31% | |||
| Tefritobasalt | 9.20 | 2.16 | 1.72 | 2.15 | 1.41 | 0.040 | 1.61 | 1.92 | 0.1575 | 10.63% | |||
| Coal mining waste | 0.58 | 0.11 | 0.02 | 0.04 | 0.01 | 0.008 | 0.25 | 0.033 | 0.1575 | 10.63% | |||
| Lead slag | 0.88 | 0.22 | 1.27 | 0.51 | 0.21 | 0.001 | 0.003 | 0.321 | 0.0508 | 3.43% | |||
| Raw mix | 13.59 | 3.28 | 3.45 | 42.48 | 2.29 | 0.13 | 32.52 | 2.27 | 1.4818 | 100.00% | |||
| By component chemical composition of the clinker | Component content | ||||||||||||
| Limestone | 4.32 | 1.16 | 0.64 | 58.95 | 0.98 | 0.112 | - | - | 66.17% | ||||
| Tefritobasalt | 13.63 | 3.20 | 2.55 | 3.19 | 2.08 | 0.060 | - | 2.845 | 14.38% | ||||
| Coal mining waste | 0.86 | 0.17 | 0.03 | 0.05 | 0.01 | 0.012 | - | 0.049 | 14.39% | ||||
| Lead slag | 1.31 | 0.33 | 1.89 | 0.75 | 0.31 | 0.002 | - | 0.476 | 5.06% | ||||
| clinker | 20.14 | 4.86 | 5.11 | 62.95 | 3.39 | 0.19 | - | 3.37 | 100.00% | ||||
| Chemical composition of raw mix and clinker | SC | n | p | TEC. (kcal/kg) | Gfuel. kg ref.fuel/t cl) | ||||||||
| Raw mix | 13.59 | 3.28 | 3.45 | 42.48 | 2.29 | 0.13 | 32.52 | 2.27 | 0.94 | 2.02 | 2.02 | - | - |
| clinker | 20.14 | 4.86 | 5.11 | 62.95 | 3.39 | 0.19 | - | 3.37 | 0.94 | 0.95 | 0.95 | 364.1 | 196 |
| Mineralogical composition of the clinker | |||||||||||||
| Minerals | 3CaO·SiO2 | 2CaO·SiO2 | 3CaO·Al2O3 | 4CaO·Al2O3·Fe2O3 | CaSO4 | MgO | |||||||
| wt.% | 57.88 | 18.82 | 6.46 | 11.61 | 0.32 | 3.39 | |||||||
| Mix | Composition of the Raw Batch, wt.% | Specific Consumption of the Raw Materials, t/t of Clinker | SC | Modules | Amount of Free CaO at 1350 °C, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lime Stone | Tefrito Basalt | Coal Mining Waste | Lead Slag | Lime Stone | Tefrito Basalt | Coal Mining Waste | Lead Slag | n | p | |||
| 1 | 66.17 | 14.38 | 14.39 | 5.06 | 1.1160 | 0.1575 | 0.1575 | 0.0508 | 0.94 | 2.02 | 0.95 | 0.2 |
| Cement | Sieve Residue,% | Specific Surface Area, cm2/g | Strength of Small Samples 2 × 2 × 2 cm, (MPa) | ||
|---|---|---|---|---|---|
| No. 02 | No. 008 | 7 Day | 28 Day | ||
| Cement | 3.3 | 13 | 3245 | 27.4 | 41.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhanikulov, N.; Sapargaliyeva, B.; Agabekova, A.; Alfereva, Y.; Baidibekova, A.; Syrlybekkyzy, S.; Nurshakhanova, L.; Nurbayeva, F.; Sabyrbaeva, G.; Zhatkanbayev, Y.; et al. Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement. J. Compos. Sci. 2023, 7, 226. https://doi.org/10.3390/jcs7060226
Zhanikulov N, Sapargaliyeva B, Agabekova A, Alfereva Y, Baidibekova A, Syrlybekkyzy S, Nurshakhanova L, Nurbayeva F, Sabyrbaeva G, Zhatkanbayev Y, et al. Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement. Journal of Composites Science. 2023; 7(6):226. https://doi.org/10.3390/jcs7060226
Chicago/Turabian StyleZhanikulov, Nurgali, Bayan Sapargaliyeva, Aktolkyn Agabekova, Yana Alfereva, Aidin Baidibekova, Samal Syrlybekkyzy, Lazzat Nurshakhanova, Farida Nurbayeva, Gulzhan Sabyrbaeva, Yergazy Zhatkanbayev, and et al. 2023. "Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement" Journal of Composites Science 7, no. 6: 226. https://doi.org/10.3390/jcs7060226
APA StyleZhanikulov, N., Sapargaliyeva, B., Agabekova, A., Alfereva, Y., Baidibekova, A., Syrlybekkyzy, S., Nurshakhanova, L., Nurbayeva, F., Sabyrbaeva, G., Zhatkanbayev, Y., Kozlov, P., Izbassar, A., & Kolesnikova, O. (2023). Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement. Journal of Composites Science, 7(6), 226. https://doi.org/10.3390/jcs7060226






