The Antioxidant Protective Effect of Iris-Squid-Derived Protein Hydrolysates (>10 kDa) in HSF Fibroblast Cells Induced by H2O2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carcass Protein from S. oualaniensis
2.3. Preparation of PHCSO, PHCSO-1 and PHCSO-2
2.4. Molecular Pattern
2.5. Amino Acid Composition
2.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.7. Determination of Antioxidant Activity
2.8. HSF Cells Culture
2.9. Effect of PHCSO-1 and PHCSO-2 on Cell Viability of HSF Cells
2.10. Establishment of H2O2-Induced Oxidative Stress Model of HSF Cells
2.11. PHCSO and PHCSO-2 Repair HSF Cells from Oxidative Stress Induced by H2O2
2.12. ROS Fluorescence Staining
2.13. Effect of PHCSO-2 on Intracellular Antioxidant and Inflammatory Factors
2.14. Statistical Analysis
3. Results
3.1. SDS-PAGE Protein Pattern
3.2. Amino Acid Composition
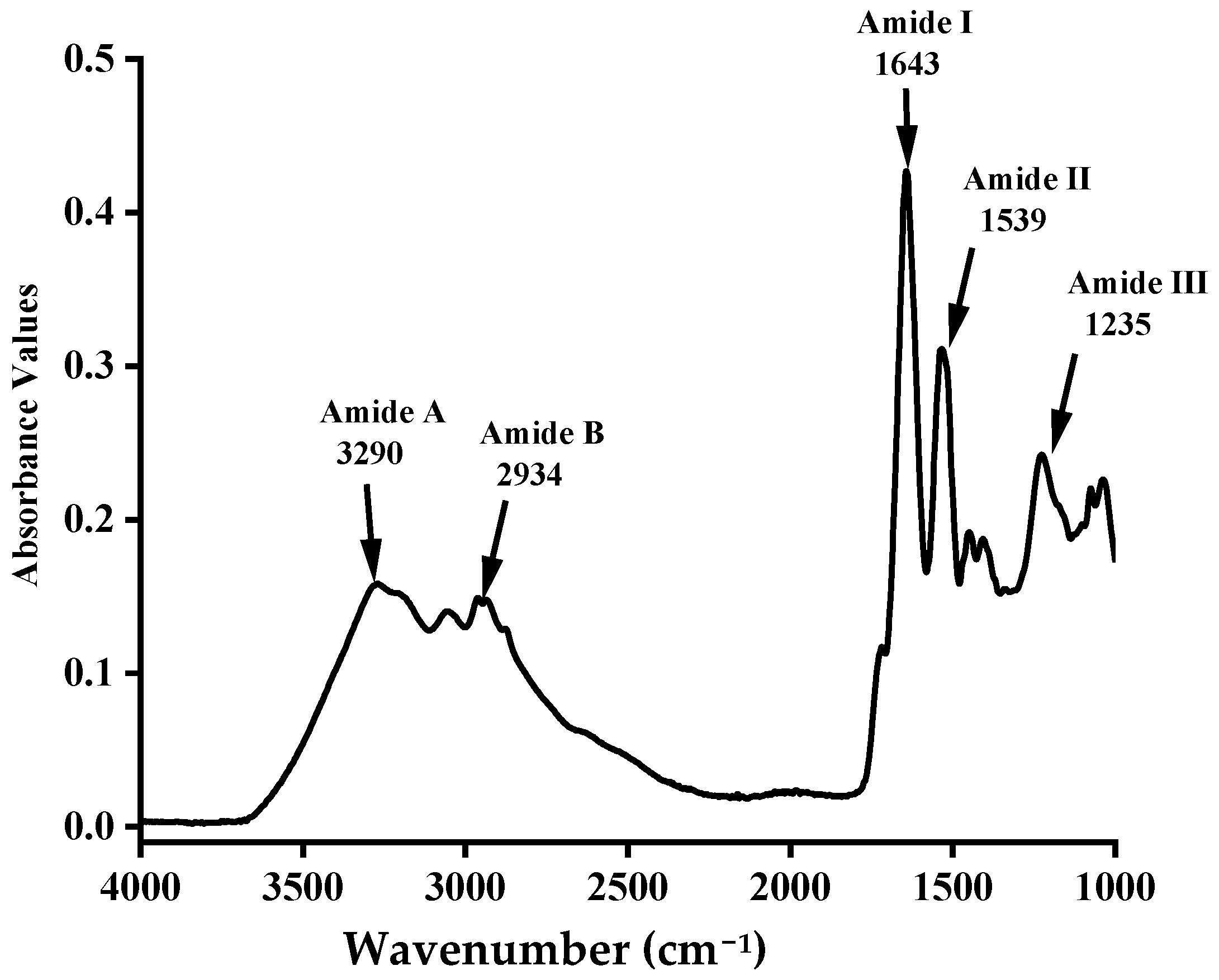
3.3. Fourier Transform Infrared (FTIR) Spectra
3.4. Analysis of Antioxidant Activity of PHCSO, PHCSO-1 and PHCSO-2
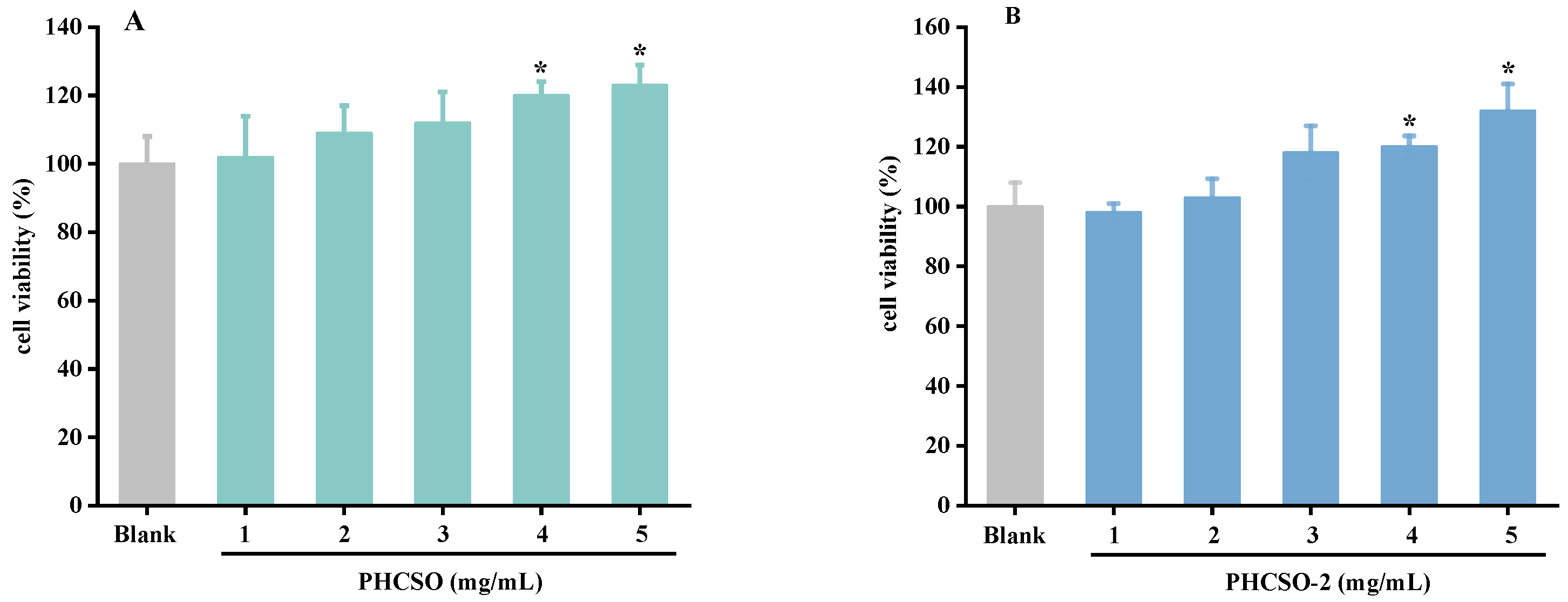
3.5. The Effect of PHCSO and PHCSO-2 on the Viability of HSF Cells
3.6. Repair Effect of PHCSO and PHCSO-2 on H2O2-Induced Oxidative Stress of HSF Cells
3.7. Cell Morphology Observation and Repair Effects
3.8. Intracellular SOD, CAT, GSH and MDA Level
3.9. The Inflammatory Factors IL-1, IL-6 and TNF-α Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vladkova, T.; Georgieva, N.; Staneva, A.; Gospodinova, D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants 2022, 11, 439. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski. 2020, 48, 124–127. [Google Scholar] [PubMed]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen Fragmentation Promotes Oxidative Stress and Elevates Matrix Metalloproteinase-1 in Fibroblasts in Aged Human Skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Topoľská, D.; Mendichi, R.; Collins, M.N.; Sasinková, V.; Šoltés, L. Hydrogen peroxide generation by the Weissberger biogenic oxidative system during hyaluronan degradation. Carbohydr. Polym. 2016, 148, 189–193. [Google Scholar] [CrossRef]
- Peng, L.; Kong, X.; Wang, Z.; Ai-Lati, A.; Ji, Z.; Mao, J. Baijiu vinasse as a new source of bioactive peptides with antioxidant and anti-inflammatory activity. Food Chem. 2021, 339, 128159. [Google Scholar] [CrossRef]
- Marcuse, R. Antioxidative Effect of Amino-Acids. Nature 1960, 186, 886–887. [Google Scholar] [CrossRef]
- López-García, G.; Dublan-García, O.; Arizmendi-Cotero, D.; Gomez-Olivan, L.M. Antioxidant and Antimicrobial Peptides Derived from Food Proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Sudhakar, S.; Nazeer, R.A. Structural characterization of an Indian squid antioxidant peptide and its protective effect against cellular reactive oxygen species. J. Funct. Foods 2015, 14, 502–512. [Google Scholar] [CrossRef]
- Singh, A.; Hong, H.; Benjakul, S. Threadfin bream surimi gel containing squid fin protein hydrolysate: Textural properties, acceptability, and volatile profile. J. Food Sci. 2022, 87, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, B.; Deng, Y.; Zhao, Y. In vitro anti-inflammatory and antioxidant activities and protein quality of high hydrostatic pressure treated squids (Todarodes pacificus). Food Chem. 2016, 203, 258–266. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Fernández-Martín, F.; Montero, P. Jumbo squid (Dosidicus gigas) myofibrillar protein concentrate for edible packaging films and storage stability. LWT-Food Sci. Technol. 2014, 55, 543–550. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Dai, H.J.; Sun, Y.L.; Zheng, X.L.; Feng, Z.X.; Wen, J.; Zhao, J.; Chen, D.H. Extraction optimization, preliminary characterization and antioxidant activity of glycoproteins from the muscle of Sepia pharaonis. Food Sci. Technol. Res. 2016, 22, 39–52. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, R.; Song, R. Extraction of Cathepsin D-Like Protease from Neon Flying Squid (Ommastrephes bartramii) Viscera and Application in Antioxidant Hydrolysate Production. Biomolecules 2019, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yao, Y.; Ibrahim, M.A.A.; Halawany, A.M.E.; Yang, L.; Zhang, X. Production of Dual Inhibitory Hydrolysate by Enzymatic Hy-drolysis of Squid Processing By-product. Mar. Biotechnol. (NY) 2022, 24, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT—Food Sci. Technol. 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Food, J.; Organisation, A. World Health Organisation Ad Hoc Expert Committee. Energy and protein requirements. FAO Nutr. Meet. Rep. Ser. 1973, 7, 105–106. [Google Scholar]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.-K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Park, M.J.; Bae, Y.S. Fermented Acanthopanax koreanum root extract reduces UVB-and H2O2-induced senescence in human skin fibroblast cells. J. Microbiol. Biotechnol. 2016, 26, 1224–1233. [Google Scholar] [CrossRef]
- Feng, B.; Ma, L.J.; Yao, J.J.; Fang, Y.; Mei, Y.A.; Wei, S.M. Protective effect of oat bran extracts on human dermal fibroblast injury induced by hydrogen peroxide. J. Zhejiang Univ. Sci. B 2013, 14, 97. [Google Scholar] [CrossRef]
- Zdanov, S.; Debacq-Chainiaux, F.; Remacle, J.; Toussaint, O. Identification of p38MAPK-dependent genes with changed transcript abundance in H2O2-induced premature senescence of IMR-90 hTERT human fibroblasts. FEBS Lett. 2006, 580, 6455–6463. [Google Scholar] [CrossRef]
- Makpol, S.; Jam, F.A.; Khor, S.C.; Ismail, Z.; Mohd Yusof, Y.A.; Ngah, W.Z. Comparative effects of biodynes, tocotrienol-rich fraction, and tocopherol in enhancing collagen synthesis and inhibiting collagen degradation in stress-induced premature senescence model of human diploid fibroblasts. Oxid. Med. Cell Longev. 2013, 2013, 298574. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of Reactive Oxygen Species in Skin Carcinogenesis. Antioxidants Redox Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Izutsu, Y.; Yahagi, S.; Okano, Y. Reactive Oxygen Species in HaCaT Keratinocytes After UVB Irradiation Are Triggered by Intracellular Ca2+ Levels. J. Investig. Dermatol. Symp. Proc. 2009, 14, 50–52. [Google Scholar] [CrossRef]
- Calabrese, V.; Calafato, S.; Puleo, E.; Cornelius, C.; Sapienza, M.; Morganti, P.; Mancuso, C. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: Role of vitagenes. Clin. Dermatol. 2008, 26, 358–363. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, Q.; Zhang, J.; Yan, S.; Zhu, L.; Zhu, B. The Protective Effect of Stauntonia Chinensis Polysaccharide on CCl4-induced Acute Liver Injuries in Mice. Int. J. Biomed. Sci. 2014, 10, 16–20. [Google Scholar] [PubMed]
- Krych-Madej, J.; Gebicka, L. Do pH and flavonoids influence hypochlorous acid-induced catalase inhibition and heme modification? Int. J. Biol. Macromol. 2015, 80, 162–169. [Google Scholar] [CrossRef]
- Xiong, Q.; Xie, P.; Li, H.; Hao, L.; Li, G.; Qiu, T.; Liu, Y. Acute effects of microcystins exposure on the transcription of antioxidant enzyme genes in three organs (liver, kidney, and testis) of male Wistar rats. J. Biochem. Mol. Toxicol. 2010, 24, 361–367. [Google Scholar] [CrossRef]
- Hanko, M.; Švorc, Ľ.; Planková, A.; Mikuš, P. Overview and recent advances in electrochemical sensing of glutathione–A review. Anal. Chim. Acta 2019, 1062, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yapislar, H.; Taskin, E. L-carnosine alters some hemorheologic and lipid peroxidation parameters in nephrectomized rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 399. [Google Scholar]
- Kiyoshima, T.; Enoki, N.; Kobayashi, I.; Sakai, T.; Nagata, K.; Wada, H.; Fujiwara, H.; Ookuma, Y.; Sakai, H. Oxidative stress caused by a low concentration of hydrogen peroxide induces senescence-like changes in mouse gingival fibroblasts. Int. J. Mol. Med. 2012, 30, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, G.; Mitchell, P.L.; Rioux, L.E.; Hasan, F.; Jin, T.; Roblet, C.R.; Doyen, A.; Pilon, G.; St-Pierre, P.; Lavigne, C.; et al. Low-Molecular-Weight Peptides from Salmon Protein Prevent Obesity-Linked Glucose Intolerance, Inflammation, and Dyslipidemia in LDLR-/-/ApoB100/100 Mice. J. Nutr. 2015, 145, 1415–1422. [Google Scholar] [CrossRef]
- Sun, X.; Chakrabarti, S.; Fang, J.; Yin, Y.; Wu, J. Low-molecular-weight fractions of Alcalase hydrolyzed egg ovomucin extract exert anti-inflammatory activity in human dermal fibroblasts through the inhibition of tumor necrosis factor–mediated nuclear factor κB pathway. Nutr. Res. 2016, 36, 648–657. [Google Scholar] [CrossRef]






| Amino Acid | Content (g/100 g) | Percentage of Total Amino Acids % | |
|---|---|---|---|
| Polar amino acid | Glycine (Gly) | 2.46 | 4.12 |
| Tyrosine (Tyr) | 2.43 | 4.06 | |
| Serine (Ser) | 2.73 | 4.58 | |
| Threonine (Thr) | 2.90 | 4.86 | |
| Nonpolar amino acid | Alanine (Ala) | 3.43 | 5.74 |
| Valine (Val) | 3.04 | 5.11 | |
| Leucine (Leu) | 5.59 | 9.38 | |
| Isoleucine (Ile) | 3.27 | 5.48 | |
| Phenylalanine (Phe) | 2.69 | 4.51 | |
| Proline (Pro) | 0.93 | 1.56 | |
| Tryptophan (Try) | 0.61 | 1.02 | |
| Methionine (Met) | 1.97 | 3.30 | |
| Amino acids with negative charged | Lysine (Lys) | 5.31 | 8.91 |
| Arginine (Arg) | 4.63 | 7.77 | |
| Histidine (His) | 1.77 | 2.97 | |
| Amino acids with positive charge | Aspartic (Asp) | 6.34 | 10.63 |
| Glutamic (Glu) | 9.54 | 16.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Diao, X.; Pu, X.; Tang, P.; Elango, J.; Wu, W. The Antioxidant Protective Effect of Iris-Squid-Derived Protein Hydrolysates (>10 kDa) in HSF Fibroblast Cells Induced by H2O2. J. Compos. Sci. 2023, 7, 228. https://doi.org/10.3390/jcs7060228
Li N, Diao X, Pu X, Tang P, Elango J, Wu W. The Antioxidant Protective Effect of Iris-Squid-Derived Protein Hydrolysates (>10 kDa) in HSF Fibroblast Cells Induced by H2O2. Journal of Composites Science. 2023; 7(6):228. https://doi.org/10.3390/jcs7060228
Chicago/Turabian StyleLi, Na, Xiaozhen Diao, Xinyi Pu, Pengjie Tang, Jeevithan Elango, and Wenhui Wu. 2023. "The Antioxidant Protective Effect of Iris-Squid-Derived Protein Hydrolysates (>10 kDa) in HSF Fibroblast Cells Induced by H2O2" Journal of Composites Science 7, no. 6: 228. https://doi.org/10.3390/jcs7060228
APA StyleLi, N., Diao, X., Pu, X., Tang, P., Elango, J., & Wu, W. (2023). The Antioxidant Protective Effect of Iris-Squid-Derived Protein Hydrolysates (>10 kDa) in HSF Fibroblast Cells Induced by H2O2. Journal of Composites Science, 7(6), 228. https://doi.org/10.3390/jcs7060228








